Background: T-lymphoblastic lymphoma (T-LBL) is a highly aggressive non-Hodgkin lymphoma seen in young adults that usually presents with a mediastinal mass and less than 20% bone marrow involvement with neoplastic cells. T-LBL and T-cell ALL are morphologically and immmunophenoytpically similar and are thus frequently grouped together. However there are differences in clinical presentation, cytogenetic features and molecular pathogenesis indicating biologic differences. Patients with relapsed refractory T-LBL have poor prognosis and there is a paucity of data on allogeneic HCT (allo-HCT) outcomes in this setting.
Methods: We retrospectively reviewed medical records of 25 consecutive patients with T lymphoblastic lymphoma without prior auto-HCT who underwent allo-HCT at City of Hope from January 2000 to June 2018 after IRB approval was obtained. Descriptive statistics were used to summarize baseline patient demographic, treatment, and disease characteristics. Kaplan-Meier curves and the log-rank test were used to evaluate the overall survival (OS) and progression-free survival (PFS). Cumulative incidences of time to relapse and time to non-relapse mortality (NRM) were calculated with relapse and NRM as competing risks. Cumulative incidences of acute and chronic GVHD were calculated as time to onset of GVHD with relapse and death as competing events for GVHD.
Results: 25 patients were included for the analysis. Median age at the time of allo-HCT was 25 years (range 7-70 years). 21 patients (80%) received myeloablative conditioning: FTBI containing regimens (n=20) with FTBI/VP-16 being most commonly used(n=16) and one patient received Bu/Cy based conditioning; 4 patients received reduced intensity conditioning with Flu/Mel. Sibling HCT was performed in 12 patients (48%), while MUD HCT was performed in 13 patients (52%) with fully matched HLA unrelated donor in 9 (36%) and HLA mismatched in 4 (16%) patients. GVHD prophylaxis consisted of tacrolimus/sirolimus (n=10), tacrolimus or cyclosporine/MTX (n=7), tacrolimus/sirolimus/MTX (n=4) and others (n=4) Source of stem cells was PBSC in 19 (76%), bone marrow in 5 (20%), and cord blood in 1 (4%) patients. At the time of allo-HCT, there were a total of 11 (44%) patients in complete remission (CR1 n=4, CR2+ n=7), 9 (36%) patients in partial remission and unknown remission status (n=5).
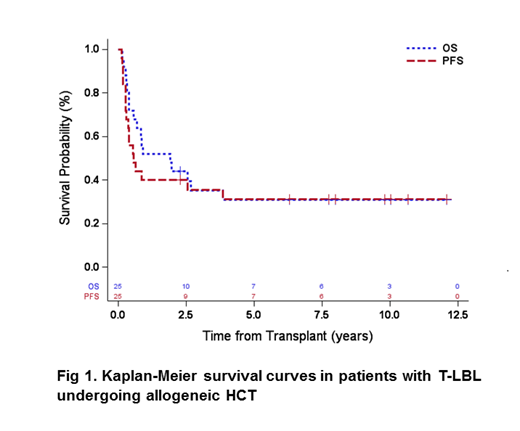
The median follow-up among survivors was 8.9 years (range 2.3-12.1). The 5- and 10-year PFS was 31% (95% CI: 14%-50%). The 5- and 10-year OS was 31% (95% CI: 14%-49%) (Fig.1). Cumulative incidence of relapses at 5 and 10 years were both 36% (95% CI: 18%-55%) while NRM at 5 and 10 years was 33% (95% CI: 15%-52%). At day 100 after allo-HCT, the rates of acute GVHD grade II-IV were 63% (95% CI: 39%-79%) and grade III-IV of 25% (95%CI: 10%-44%). Chronic GVHD rates at 3 years were 60% (95% CI: 37%-77%). In univariate analysis, preHCT remission status did not improve PFS on long term follow up.
Conclusions: Our results in patients with T-LBL shows 5-year PFS and OS of 31%, respectively, indicating that a subset of patients can be cured with allo-HCT in the high-risk setting.
Salhotra:Celgene: Other: Research Support; Kadmon Corporation: Other: Non paid consultant. Popplewell:City of Hope: Employment. Herrera:Kite Pharma: Consultancy, Research Funding; Merck: Consultancy, Research Funding; Genentech, Inc.: Consultancy, Research Funding; Pharmacyclics: Research Funding; AstraZeneca: Research Funding; Immune Design: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Adaptive Biotechnologies: Consultancy; Gilead Sciences: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding. Mei:Seattle Genetics, Inc.: Research Funding. Aldoss:AUTO1: Consultancy; Helocyte: Consultancy, Honoraria, Other: travel/accommodation/expenses; Jazz Pharmaceuticals: Honoraria, Other: travel/accommodation/expenses, Speakers Bureau; Agios: Consultancy, Honoraria. Zain:Seattle Genetics: Honoraria, Speakers Bureau; spectrum: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal