Background:
In RRMM, the median overall survival (OS) of pts with RRMM who progressed after exposure to ≥3 prior therapies is ~13 mo, indicating a high unmet need. LCAR-B38M is a structurally differentiated CAR-T cell therapy containing a 4-1BB co-stimulatory domain and 2 BCMA-targeting single-domain antibodies designed to confer avidity. Earlier results from LEGEND-2 (NCT03090659), a first-in-human phase 1 study using LCAR-B38M CAR-T cells in 74 pts with RRMM conducted in 4 hospitals in China (Jiangsu Provincial People's Hospital; Ruijin Hospital; Changzheng Hospital; and the Second Affiliated Hospital of Xi'an Jiaotong University), showed encouraging efficacy and manageable safety. Key eligibility criteria included RRMM with ≥3 prior lines of therapy. Here, we present long-term follow-up data on safety and efficacy from the Xi'an site.
Methods:
In the Xi'an site-specific protocol (n=57), lymphodepletion was performed using cyclophosphamide (Cy; 300 mg/m2)alone for 3 days. LCAR-B38M (median CAR+ T cells, 0.5×106 cells/kg; range, 0.07-2.1 × 106) was infused in 3 split infusions. The primary objective was to evaluate the safety of LCAR-B38M; the secondary objective was to evaluate anti-myeloma response of treatment. Adverse events (AEs) were graded using the NCI-CTCAE v4.03, cytokine release syndrome (CRS) was assessed per Lee et al. 2014, and response was evaluated using IMWG criteria.
Results:
As of the 12/31/18 cutoff date (median follow-up, 19 mo; 95% confidence interval [CI], 17-22), enrollment at Xi'an is complete, and 57 pts have been infused with LCAR-B38M.
AEs were reported by all pts: pyrexia (91%), CRS (90%), thrombocytopenia (49%), and leukopenia (47%). Grade ≥3 AEs were reported by 65% of pts: leukopenia (30%), thrombocytopenia (23%), and increased aspartate aminotransferase (21%). CRS was mostly grade 1 (47%) and 2 (35%); 4 pts (7%) had grade 3 events; no grade 4/5 CRS was observed. Neurotoxicity was observed in 1 pt (grade 1 aphasia, agitation, seizure-like activity). The median time to onset of CRS was 9 days (range, 1-19) with a median duration of 9 days (range, 3-57); all but 1 CRS events resolved.
Peak levels of LCAR-B38M (≥1x104 copies/µg genomic DNA) were observed in a majority of pts with blood samples for analysis (n=32). LCAR-B38M was not detectable in peripheral blood in 71% of pts at 4 mo; 5 pts showed CAR-T cell persistence for up to 10 months.
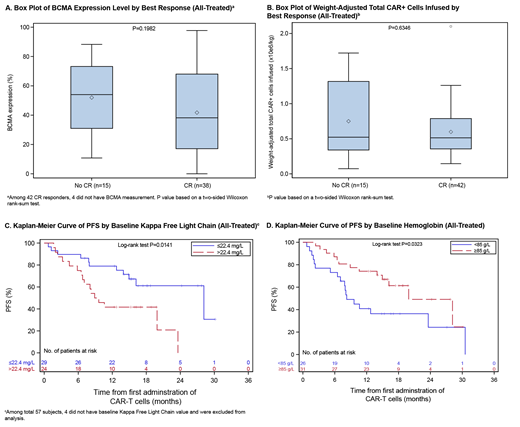
The overall response rate (partial response [PR] or better) was 88% (95% CI, 76-95), complete response (CR) was achieved by 42 pts (74%; 60-85), very good partial response (VGPR) by 2 pts (4%; 0.4-12), and PR by 6 pts (11%; 4-22). Of pts with CR, 39/42 were minimal residual disease negative (MRD-neg, 8-color flow cytometry). The median time to first response was 1.2 mo. There was no relationship between best response and baseline BCMA expression level or weight-adjusted CAR+ cells infused (Fig 1a,b).
At cutoff, the median follow-up was 19 mo [17-22]. Median OS has not yet been reached. The OS rate at 18 mo was 68% (54─79) with a median duration of response (mDOR) of 22 mo (13-29). Of MRD-neg pts with CR, 91% (75-97) are still alive at data cut, with a 27 mo (16-NE) mDOR.
Overall, 26 (46%) of 57 all-treated pts and 25 (64%) of 39 MRD-neg pts with CR remain progression-free. The median progression-free survival (PFS) for all-treated pts was 20 mo (10-28); median PFS for MRD-neg pts with CR was 28 mo (20-31). At 18 months, the PFS rate was 50% (36-63) for all pts and 71% (52-84) for MRD-neg pts with CR. Factors contributing to long-term response are shown in Fig 1c,d.
Seventeen pts died during the study and the follow-up period: progressive disease (PD; n=11), disease relapse, PD + lung infection, suicide after PD, esophageal carcinoma, infection, and pulmonary embolism and acute coronary syndrome (n=1 each). Of these, 4 did not achieve PR or better; 1 was not evaluable.
Conclusions:
This study provides evidence that LCAR-B38M is a highly effective therapy for RRMM, regardless of baseline BCMA expression. LCAR-B38M displayed a manageable safety profile consistent with its known mechanism of action and, with a median follow-up of 19 months, demonstrated deep and durable responses in pts with RRMM. A phase 1b/2 clinical study is ongoing in the United States (CARTITUDE-1, NCT03548207, JNJ-68284528), and a phase 2 confirmatory study has initiated in China (CARTIFAN-1, NCT03758417).
Zhuang:Nanjing Legend Biotech: Employment. Fan:Legend Biotech: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal