
Introduction
Elderly non-transplant eligible newly diagnosed multiple myeloma (nte-NDMM) patients also benefit from novel therapies, however, overall survival (OS) is inferior in unfit and frail compared to fit patients as defined by the International Myeloma Working Group (IMWG) frailty index. This is caused by a high discontinuation rate due to toxicity. Therefore, a less toxic effective treatment for unfit and frail patients is needed. In view of the favorable safety profile of ixazomib (Ixa) and daratumumab (Dara), we investigated the efficacy and feasibility of treatment with Ixa and Dara plus low dose dexamethasone (Ixa-Dara-dex) in unfit and frail patients. This trial was registered at www.trialregister.nlwww.trialregister.nl as NTR6297.
Methods
In this prospective multicenter phase II trial, treatment consisted of nine 28 day-induction cycles consisting of Ixa 4 mg (days 1, 8, 15), Dara 16 mg/kg (cycle 1-2: days 1, 8, 15, 22; cycle 3-6: days 1, 15; cycle 7-9: day 1) and dex (in combination with Dara; cycle 1-2: 20 mg; subsequent cycles 10 mg) followed by maintenance therapy with Ixa (days 1, 8, 15, 29, 36, 43) and Dara (day 1) of 8-week cycles, until progression for a maximum of 2 years. A pre-specified efficacy analysis was planned for the first eligible 23 unfit and 23 frail patients separately at the time the data of the first 9 cycles induction therapy was available.
Inclusion criteria were unfit or frail NDMM patients according to the IMWG frailty index. Main exclusion criteria were severe cardiac dysfunction, chronic obstructive pulmonary disease with an FEV1 <50% of expected and a creatinine clearance of <20 ml/minute.
We here report the overall response rate (ORR) on induction treatment, progression free survival (PFS) and OS, treatment discontinuation and toxicity of the first 23/65 eligible unfit and 23/65 frail patients during induction therapy. In addition, we present the mortality rate for all patients who were included in the study (65 unfit, 67 frail), with data cut-off June 18, 2019.
Results
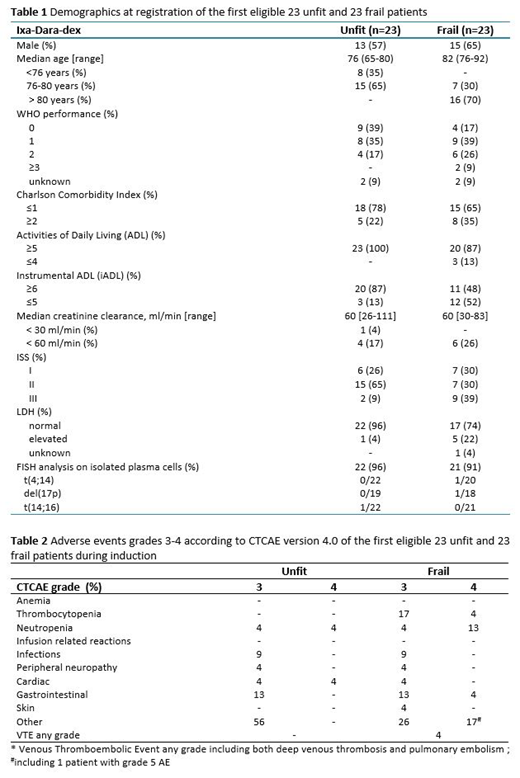
The demographics of the first 23 unfit and 23 frail patients are described in Table 1. Median follow-up of these first 23 unfit and 23 frail patients is 12.7 months (range 9.1-18.3) and 13.4 months (range 9.2-17.7), respectively.
ORR during induction was 87% in unfit (48% partial response [PR] and 39% very good partial response [VGPR]) and 78% in frail (48% PR, 26% VGPR, 4% stringent complete response). Nine months PFS rates were 78% (95% Confidence Interval [CI] 55-90) and 61% (95% CI 38-77), respectively. Nine months OS rates were 100% and 83% (95% CI 60-93), respectively.
Sixteen/23 (70%) unfit and 14/23 (61%) frail patients completed induction treatment with Ixa-Dara-dex. Reasons for treatment discontinuation were progressive disease (PD, n=5), toxicity (n=1) and incompliance (n=1) in unfit and intercurrent death (n=3, all within 3 cycles), PD (n=2), incompliance (n=2) and other reasons (n=2) in frail. The median and inter-quartile range of relative dose intensity (RDI) for respectively unfit and frail were 0.96 and 0.91 for Ixa, 0.98 and 0.96 for Dara and 1.0 and 0.99 for dex.
Toxicity is described in Table 2. Hematological toxicity was limited, being mainly neutropenia (in unfit both 4% grade 3 and 4; in frail 4% grade 3 and 13% grade 4) and thrombocytopenia occurring only in frail (17% grade 3, 4% grade 4). Non-hematological toxicity was manageable, with grade 3 infections occurring in 9% of both unfit and frail patients. In both arms, there were no infusion related reactions and only 4% grade 3 neuropathy was reported.
Additionally, we investigated the mortality rate of all included 65 unfit and 67 frail patients, with a limited follow-up of 7.1 months (range 1-18.3) and 8.8 months (range 0.4-17.7), respectively. The mortality rate was only 2% in unfit (1/65 due to PD). Thirteen/67 (19%) of frail patients died, which was caused by infections (n=6; 3 pneumonia, 1 influenza B, 1 erysipelas), sudden death (n=2), organ dysfunction (n=2), incompliance (n=1) and PD (n=2). Early death rate (≤3 months of registration) was 0% in unfit and 8/67 (12%) in frail.
Conclusion
Ixa-Dara-dex is an effective therapeutic regimen in unfit patients with limited toxicity, not giving rise to (early) mortality. Additionally, in the majority of frail patients this regimen is active and feasible. However, better identification and support of those patients is warranted, as we observed early mortality due to vulnerability and infections.
Van De Donk:Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; AMGEN: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees. Sonneveld:SkylineDx: Research Funding; Takeda: Honoraria, Research Funding; Karyopharm: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; BMS: Honoraria; Amgen: Honoraria, Research Funding. Levin:Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Educational grant ; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Educational Grant; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Educational Grant; Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: Educational Grant; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Educational grant . Zweegman:Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal