Introduction
Andexanet alfa is a modified recombinant inactive form of human factor Xa developed for reversal of factor Xa inhibitors. In the ANNEXA-4 study, patients with acute major bleeding within 18 h after administration of a factor Xa inhibitor were enrolled and received a bolus of andexanet, followed by a 2-h infusion (Connolly, NEJM 2019;380:1326). In this study, 82% of patients achieved effective hemostasis at 12 h and 10% developed a thrombotic event within 30 days. Anti-Xa activity decreased by 92% after the andexanet bolus but partially recovered after the end of the 2 h infusion. In the present analysis, we evaluated the effect of andexanet alfa on thrombin generation (TG) in patients enrolled in the ANNEXA-4 study and we explored whether TG predicts effective hemostasis or thrombotic events.
Methods
We included all patients who received andexanet alfa. TG was expressed as the endogenous thrombin potential (ETP) which is the area under the thrombin generation curve. We plotted mean TG at different timepoints between baseline and 30 days after andexanet alfa in patients treated with apixaban and rivaroxaban. We compared the absolute ETP level at 8 h (ETP-8H) after andexanet bolus as this was the first timepoint after the 2 h infusion for which an ETP level was available for most patients. We compared ETP-8H levels between patients with and without effective hemostasis and between those with and without thrombotic events, respectively. ETP-8H was evaluated as a predictor of effective hemostasis and thrombotic events by logistic regression analysis in all patients, and in subgroups of patients with intracranial hemorrhage (ICH) and non-ICH separately. In the ICH subgroups, ETP-8H was also evaluated as a predictor of absolute change in hematoma volume.
Results
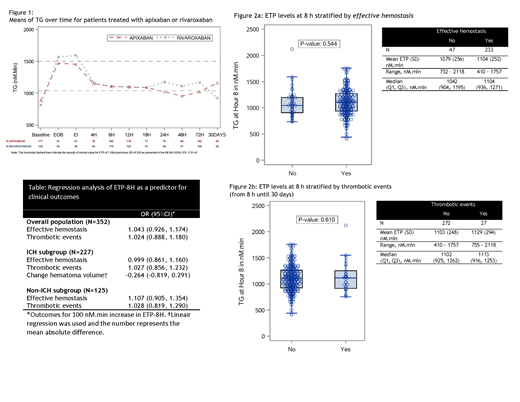
The study population comprised 352 patients (mean age 77.4 years; 47% female) with acute major bleeding (64% ICH, 26% gastrointestinal, 10% other) treated with apixaban (55%), rivaroxaban (36%), enoxaparin (6%), or edoxaban (3%). ETP-8H was available for 327 patients (93%). In patients treated with apixaban or rivaroxaban, andexanet bolus promptly increased mean ETP and this was maintained during infusion. After end of infusion ETP fell but remained in the reference range for at least 18 hours (Figure 1). ETP-8H was similar in patients with or without effective hemostasis (Fig 2a, p = 0.544) and in patients with or without thrombotic complications (Fig 2b, p = 0.610). In the logistic regression analysis, ETP-8H did not predict effective hemostasis (p=0.491) or thrombotic events (p=0.743) (Table), and these results were consistent in ICH and non-ICH patients. ETP-8H did not predict hematoma growth in patients with ICH (p = 0.349).
Conclusion
A bolus of andexanet alfa, followed by a 2-h infusion in patients with factor Xa inhibitor associated major bleeding promptly restores thrombin generation and this effect is sustained for at least 18 hours. Thrombin generation at 8 h after andexanet bolus did not predict effective hemostasis, intracranial hematoma growth, or thrombotic events. This may be explained by the andexanet dose which was chosen to ensure full reversal of the factor Xa inhibitor in all patients.
Coppens:Bayer: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria; Daiichi Sankyo: Honoraria, Research Funding; Sanquin Blood Supply: Research Funding; Pfizer: Honoraria; Uniqure: Research Funding; CSL Behring: Honoraria, Research Funding; Portola Pharmaceuticals, Inc: Honoraria; Boehringer Ingelheim: Research Funding. Middeldorp:Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Other: honoraria for advisory activities; Aspen: Research Funding; Portola Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Other: honoraria for advisory activities; Boehringer Ingelheim: Membership on an entity's Board of Directors or advisory committees, Other: honoraria for advisory activities; Bayer: Membership on an entity's Board of Directors or advisory committees, Other: honoraria for advisory activities, Research Funding; Sanofi: Speakers Bureau; Daiichi Sankyo: Other: honoraria for advisory activities, Research Funding. Verhamme:Portola Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer Healthcare: Consultancy, Research Funding, Speakers Bureau; Boehringer Ingelheim: Consultancy, Research Funding, Speakers Bureau; Daiichi Sankyo: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Research Funding, Speakers Bureau; Leo Pharma: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy. Eikelboom:Heart and Stroke Foundation: Research Funding; Sanofi Aventis: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Glaxo Smith Kline: Honoraria, Research Funding; Eli Lilly: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Boehringer Ingelheim: Honoraria, Research Funding; Bayer: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding. Crowther:Bayer: Other: Data and Safety Monitoring Board, Research Funding, Speakers Bureau; BMS Canada: Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier Canada: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Other: preparing educational material and/or providing educational presentations; CSL Behring: Other: preparing educational material and/or providing educational presentations; Diagnostica Stago: Other: preparing educational material and/or providing educational presentations, Research Funding; Alnylam: Equity Ownership; Asahi Kasei: Membership on an entity's Board of Directors or advisory committees; Alexion: Speakers Bureau; Shionogi: Membership on an entity's Board of Directors or advisory committees; Octapharma: Membership on an entity's Board of Directors or advisory committees. Lu:Portola Pharmaceuticals: Employment, Equity Ownership. Yue:Portola Pharmaceuticals: Employment, Equity Ownership. Conley:Portola Pharmaceuticals, Inc.: Employment, Equity Ownership. Connolly:Portola Pharmaceuticals: Consultancy, Research Funding; Bayer Healthcare: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal