Introduction: Chronic immune thrombocytopenia (cITP) is a common, acquired, autoimmune disorder resulting in abnormally low blood platelet counts (<100×109/L) that can lead to excessive bruising and bleeding. Management of cITP includes corticosteroids as first-line therapy. However, debate exists surrounding the clinically optimal nature and order of second- and third-line treatments, including rituximab, a clinically broadly-used off-label treatment, and thrombopoietin receptor agonists (TPO-RAs; only eltrombopag and romiplostim data were available), which are approved by the US Food and Drug Administration for the treatment of cITP. We compared the risks of long-term complications (i.e., infections, thromboembolic events, and cancer) as well as mortality with TPO-RAs versus rituximab among cITP-diagnosed US Veterans who had failed first-line corticosteroids.
Methods: This historical cohort study used national US Veteran's Health Administration (VHA) datasets to identify cITP-diagnosed Veterans (ICD-9: 287.30, 287.31, 287.39; ICD-10: D69.3, D69.49) newly initiating either a TPO-RA or rituximab after prior corticosteroid use between 2011 and 2017. We controlled for selection bias and confounding by indication with inverse probability of treatment weights. Covariates included baseline demographics, platelet measures, comorbidities, and key concomitant medications. We used Cox proportional hazards regression models to calculate hazards ratios (HR) for incident complications and death associated with new users of TPO-RAs compared to new users of rituximab in the treatment-weighted population.
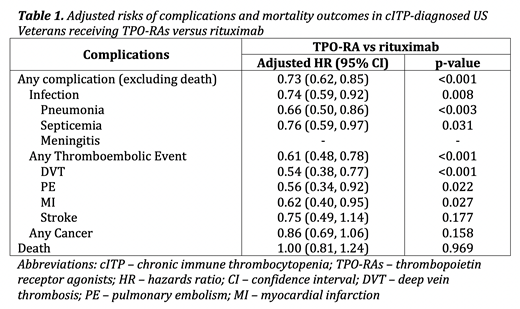
Results: Of 31,501 Veterans with a diagnosis for cITP, 429 received TPO-RA (n=244) or rituximab (n=185) as their second treatment for cITP during the study period. The mean age was 67.9 (standard deviation 12.6), 93% were male, and 14% and 74% were Black and Caucasian, respectively. After weighting, standardized differences in proportions between the groups were less than 0.1 for all baseline characteristics, indicating no significant differences between the groups among all baseline characteristics. Table 1 shows the adjusted HRs for the differences in risks of complications and death for the TPO-RAs versus the rituximab group. Patients who received TPO-RAs were 27% less likely to have complications compared to those who received rituximab (HR 0.73; 95% CI 0.62, 0.85). However, there was no observed difference in the risk of death between the groups.
Conclusions: Among cITP-diagnosed US Veterans, TPO-RA use was associated with lower risks of complications compared to rituximab. There was no observed difference in the risk of death between the groups.
Ruiz-Negron:Novartis Pharmaceuticals: Research Funding. Crook:Novartis Pharmaceuticals: Research Funding. Patwardhan:Novartis Pharmaceuticals: Employment. Said:Novartis Pharmaceuticals: Employment, Other: Restricted stocks in Novartis. Desai:Precision Xtract: Employment. LaFleur:Novartis Pharmaceuticals: Research Funding.
On-label indications for TPO-RAs (including eltrombopag and romiplostim), but off-label use of rituximab; all medications were evaluated in the treatment of chronic immune thrombocytopenia.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal