Abstract
Obesity has become a major threat to health worldwide. The prevalence of obesity is rapidly increasing, so much so that the World Health Organization has declared obesity as a global epidemic. Obesity is associated with multiple health problems, including venous thromboembolism and atrial fibrillation, both of which are treated with anticoagulation. However, obesity and treatments for obesity such as bariatric surgery can influence absorption, excretion, pharmacokinetics, and pharmacodynamics of various anticoagulants. This results in uncertainty regarding the best antithrombotic strategies in this population, particularly in the morbidly obese. In the recent years, several studies have attempted to investigate anticoagulation use in this population and provided more insight. Herein, we present 4 cases of anticoagulant use in the obese to illustrate the common challenges faced by clinicians and discuss our approach. Whenever possible, we provide a review of the literature and base our recommendations on the best available evidence.
Introduction
Obesity is a rapidly increasing health problem in the world. The National Health and Nutrition Examination Survey (NHANES) showed that the prevalence of obesity was 39.8% among US adults in 2015 to 2016.1 Similarly, close to 30% of adults in Canada are obese.2 In 2016, it was estimated that over 1.9 billion adults were overweight and over 650 million were obese globally.3 A recent study projected that by 2030, 1 in 2 adults will have obesity (body mass index [BMI] ≥ 30 kg/m2) and 1 in 4 will have severe obesity (BMI ≥ 35 kg/m2).4 Obesity is a known risk factor for venous thromboembolism (VTE), atrial fibrillation (AF), and cardiovascular diseases. The Multiple Environmental and Genetic Assessment (MEGA) study, a population-based case cohort study involving 3834 cases and 4683 controls in The Netherlands, revealed that people with BMI ≥30 kg/m2 had a twofold to threefold increased risk of VTE compared with controls with a normal BMI (22.5-25 kg/m2).5 Various mechanisms for the association between obesity and VTE have been proposed, including increased procoagulant factors such as factor VIII and fibrinogen, platelet and endothelial dysfunction, hypofibrinolysis, venous stasis, and increased inflammation.6,7 Furthermore, adipose tissue secretes hormones such as leptin, resistin, and cytokines that upregulate the expression of tissue factor, resulting in a prothrombotic state.8 In addition to the increased risk of first VTE, obesity was also associated with an increased risk of recurrent VTE in the Austrian Study on Recurrent Venous Thromboembolism.9 Moreover, obesity (BMI ≥30 kg/m2) is a factor predicting VTE recurrence in the validated prediction model “MEN continues and HERDOO2.”10,11 As for AF, a meta-analysis including 123 249 patients showed that obese individuals (most studies with cutoff BMI ≥30 kg/m2) had 49% increased risk of AF compared with nonobese counterparts.12 Although the association of obesity and AF is multifactorial, common explanations include the increased left atrial volume and size, as well as increased left ventricle diastolic dysfunction.13 Moreover, obesity is strongly associated with nonalcoholic fatty liver disease (NAFLD), which is linked to increased levels of many prothrombotic factors as well as increased incidence of cardiovascular diseases and VTE.14
In addition, obesity can affect drug pharmacokinetics (PK) by increasing volume of distribution, enhancing drug clearance, and other pharmacodynamic (PD) effects.15 Traditional anticoagulants are dosed either by laboratory testing (vitamin K antagonists [VKA]) or by weight (heparin or low-molecular-weight-heparin [LMWH]). Even for VKA, where dose is modulated to achieve a goal International Normalized Ratio (INR), weight has been shown to affect required dose.16 Therefore, when fixed-dose direct oral anticoagulants (DOACs) became the mainstay of anticoagulation, many clinicians were concerned about their efficacy and safety in patients with extreme weight. In this review, we discuss 4 common scenarios of anticoagulation management in obese patients with critical review of available literature and recent advances.
Case 1: anticoagulation for acute VTE in a morbidly obese patient
A 48-year-old man presented with a new onset of right lower-extremity pain and swelling. Doppler ultrasound showed an acute deep vein thrombosis (DVT) involving the right common femoral, femoral, popliteal, and posterior tibial veins. The patient denied recent surgery, long travel, immobilization, or trauma. Other medical history included morbid obesity with weight of 140 kg (BMI of 48 kg/m2), hypertension, hyperlipidemia, and type II diabetes. His kidney function was normal with an estimated creatinine clearance of 90 mL/min by Cockcroft-Gault formula. What anticoagulant should be recommended for this individual?
Case 2: anticoagulation for AF in a morbidly obese patient
A 76-year-old woman with hypertension and diabetes was brought to the emergency room with palpitations, bilateral lower-extremity swelling, and shortness of breath. Upon evaluation, she was found to have a new onset of AF with rapid ventricular rate as well as congestive heart failure with an ejection fraction of 35%. Her estimated creatinine clearance was 50 mL/min by Cockcroft-Gault formula. Workup and multiple medical interventions were initiated, and anticoagulation was recommended, given her high CHA2DS2-VASc (congestive heart failure, hypertension, age [>65 years = 1 point, >75 years = 2 points], diabetes, previous stroke/transient ischemic attack vascular disease, sex [2 points]) score of 6. Her weight is 125 kg with a BMI of 41 kg/m2. What anticoagulant would you recommend for this patient?
Evidence for DOAC use in the obese for VTE and AF
In recent years, DOACs have become the preferred anticoagulant for both VTE and AF for the general population.17,18 However, the efficacy and safety of DOACs in a few selected populations remain controversial, including in morbidly obese patients. To date, there are no randomized controlled trials (RCTs) to evaluate the use of DOACs compared with traditional anticoagulants in the morbidly obese population. Given the paucity of high-quality data, in 2016, the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis (ISTH) suggested against using DOACs in patients >120 kg or BMI >40 kg/m2.19 They further suggested that if DOACs were to be used in this population, drug-specific peak and trough anti-Xa levels should be monitored. These recommendations have been controversial given the lack of strong evidence for the weight and BMI cutoff. In addition, the appropriate “on-therapy” anti-Xa or drug levels as well as their correlation with clinical outcomes are unknown, and DOAC levels are not routinely available in most clinical practices. Therefore, many morbidly obese patients have been treated with DOACs without monitoring. Since this guidance statement was issued, several subgroup analyses of large prospective studies as well as recent retrospective studies have been published and provided additional important information to guide our use of DOACs in this population.
PK studies
Given the concern for altered absorption and metabolism of DOACs in obese patients, multiple studies evaluating the pharmacological profiles of various DOACs in the obese have been published. In studies of healthy volunteers, a single dose of 10 mg of rivaroxaban was given to 36 people divided into 3 weight groups: low (≤50 kg), normal (70-80 kg), and high (>120 kg). Maximal drug concentration (Cmax) and area under the drug concentration-time curve (AUC) were similar in the high weight compared with normal weight group.20 Inhibition of factor Xa activity by rivaroxaban was not affected by weight. In a similar study with apixaban involving 54 healthy volunteers, a single 10-mg dose of apixaban resulted in a 31% lower Cmax and 23% lower AUC in people with weight ≥120 kg when compared with the reference weight group (65-85 kg).21 The authors conclude that the differences are modest and dose adjustment is not necessary based on weight alone.
In studies of obese patients, some have found weight to have modest effects on DOAC levels whereas others found little effect. In the RE-LY trial, patients with weight ≥100 kg had a 21% lower dabigatran trough concentration compared with those with a weight of 50 to 100 kg.22 Although dabigatran trough concentration was associated with bleeding and thrombotic outcomes, weight was not an independent risk factor for these clinical outcomes. In another study, Piran et al measured peak plasma concentration of DOACs in 38 patients who were over 120 kg.23 They showed that only 2 patients (5%) had peak levels below the median trough concentration obtained from nonobese population, but 8 (21%) had peak concentration below the usual on-therapy range. Wasen et al found that rate of change in anti-Xa level and AUC within 4 hours after dose were significantly lower in patients with weight >120 kg compared with those with weight ≤120 kg.24 On the other hand, other studies revealed little effect of weight on drug levels. In the ENGAGE-AF TIMI 48 trial, the trough edoxaban and anti-Xa levels, as well as the effects of edoxaban vs warfarin in bleeding or thrombotic outcomes, were consistent across BMI groups.25 Barsam et al evaluated rivaroxaban levels in patients on rivaroxaban for VTE treatment or prevention, and found weight to have little effect on the PK profile, however, only 6 patients had BMI ≥40 kg/m2 in this study.26
The available PK data from these studies showed that body weight or BMI had either no or modest effects on DOAC concentration and anti-Xa levels, and whether this effect translates into pertinent clinical outcomes remains unclear. The small sample size of the morbidly obese as well as differences in the DOAC agent studied are also limitations of these data.
Prospective studies
Multiple large phase 3 prospective RCTs have demonstrated the efficacy and safety of DOACs in the treatment of VTE or AF, and subanalyses of these studies have not shown compromised efficacy or safety in the obese population.27-31 Two meta-analyses of these RCTs concluded that there were no differences in the efficacy and safety in DOACs compared with VKA in patients with high body weight.32,33 In addition, high-body-weight AF patients have a paradoxically reduced risk of thromboembolism when compared with non–high-body-weight patients, regardless of the type of anticoagulant.33 These prospective studies had the advantage of enrolling a large number of patients and were randomized. They therefore had a low risk of bias and reliable follow-up. However, they were limited by the variable definition of “high body weight,” with a cutoff of no more than 100 kg (Table 1), which provides limited applicability to the morbidly obese population addressed in the ISTH guidance (weight ≥120 kg and/or BMI ≥40 kg/m2).
Summary of prospective randomized studies in VTE
| Study . | DOAC . | Obesity weight cutoff, kg . | Study duration, mo . | n, obesity/N, total population (%) . | Efficacy outcome, % . | Safety outcome, %* . | ||
|---|---|---|---|---|---|---|---|---|
| DOAC . | VKA . | DOAC . | VKA . | |||||
| RECOVER (I/II)27 | Dabigatran | >100 | 6 | 832/5107 (16.3) | 4.1 | 3.6 | N/A | |
| EINSTEIN (DVT/PE)28,29 | Rivaroxaban | >90 | 12 | 2332/8281 (28.2) | 2.0 | 1.8 | 8.5 | 8.7 |
| AMPLIFY30 | Apixaban | ≥100 | 6 | 1017/5244 (19.4) | 2.2 | 3.5 | 0.19 | 1.9 |
| HOKUSAI31 | Edoxaban | >100 | 12 | 1265/8240 (15.4) | 3.6 | 3.5 | 8.8 | 8.3 |
| Study . | DOAC . | Obesity weight cutoff, kg . | Study duration, mo . | n, obesity/N, total population (%) . | Efficacy outcome, % . | Safety outcome, %* . | ||
|---|---|---|---|---|---|---|---|---|
| DOAC . | VKA . | DOAC . | VKA . | |||||
| RECOVER (I/II)27 | Dabigatran | >100 | 6 | 832/5107 (16.3) | 4.1 | 3.6 | N/A | |
| EINSTEIN (DVT/PE)28,29 | Rivaroxaban | >90 | 12 | 2332/8281 (28.2) | 2.0 | 1.8 | 8.5 | 8.7 |
| AMPLIFY30 | Apixaban | ≥100 | 6 | 1017/5244 (19.4) | 2.2 | 3.5 | 0.19 | 1.9 |
| HOKUSAI31 | Edoxaban | >100 | 12 | 1265/8240 (15.4) | 3.6 | 3.5 | 8.8 | 8.3 |
N/A, not available.
Safety outcome included major bleeding and clinically relevant nonmajor bleeding, except for AMPLIFY study, where only major bleeding was reported.
Since the publication of the ISTH guidance document, more post hoc analyses of phase 3 RCTs have been published focusing on the morbidly obese (Table 2). In VTE studies, the EINSTEIN DVT/pulmonary embolism (PE) pool analysis revealed that there was no association between weight or BMI and thrombotic or bleeding outcomes in patients who received rivaroxaban.34 Of 8271 patients in the pooled analysis, 303 patients (3.7%) had weight ≥120 kg, and the rate of recurrent VTE was comparable in this group with either anticoagulant (rivaroxaban, 3 of 159 [1.9%] vs VKA, 4 of 144 [2.8%]).34 In AF studies, the ARISTOTLE trial showed that in 982 patients with weight over 120 kg (5.4%), the risks of stroke events or systemic embolism and major bleeding were comparable in patients receiving apixaban vs VKA.35 In ARISTOTLE, the smaller number of patients over 140 kg (N = 258) had a risk of stroke events or systemic embolism that was numerically higher in the apixaban group, but this was not statistically significant. Similarly, the RE-LY study enrolled 1811 patients with BMI >36 kg/m2 (10%) and the ENGAGE-AF TIMI 48 study enrolled 1149 patients with BMI ≥40 kg/m2 (5.5%), and both showed comparable efficacy and safety with either dabigatran or edoxaban, respectively, compared with VKA in patients with high BMI.25,36 These post hoc analyses revealed that with over 2000 AF patients with weight > 120 kg and/or BMI > 40 kg/m2 studied, there was no evidence of inferior efficacy or safety with DOACs compared with VKA. Fewer data are available for VTE patients as only the EINSTEIN study provided further analysis in the morbidly obese population, but the results were consistent.
Summary of post hoc analysis of prospective randomized studies
| Study . | DOAC . | Anticoagulation indication . | Obesity definition . | Follow‐up duration, y . | n, obesity/N, total population . | Efficacy outcome . | Safety outcome . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DOAC . | VKA . | P and/or HR (95% CI) . | DOAC . | VKA . | P and/or HR (95% CI) . | ||||||
| RE-LY36 | Dabigatran | AF | BMI >36 kg/m2 | 1 | 1811/18113 (10%) | 0.9%* | 1.3% | P = .6 | 4.4%* | 3.7% | P = .55 |
| 1.2%† | 3%† | ||||||||||
| ARISTOTLE35 | Apixaban | AF | Weight >120 kg | 1 | 982/18139 (5.4%) | 0.44% | 1.13% | 0.39 (0.12-1.22) | 1.55% | 2.08% | 0.74 (0.37-1.50) |
| ENGAGE-AF TIMI 4825 | Edoxaban | AF | BMI ≥40 kg/m2 | 2.8 (median) | 1149/21028 (5.5%) | 2.2%§ | 1.4%§ | 1.37 (0.37-5.05)§ | 6.7%§ | 8.2%§ | 0.92 (0.54-1.57)§ |
| 3.2%ǁ | 1.4%ǁ | 2.01 (0.58-6.97)ǁ | 4.1%ǁ | 8.2%ǁ | 0.47 (0.26-0.85)ǁ | ||||||
| EINSTEIN34 (DVT/PE) | Rivaroxaban | VTE | Weight ≥100 kg | 1 | 1393/8271 (16.8%) | 2.3% | 2.0% | 1.12 (0.55-2.30) P = .25 | 0.9% | 1.2% | 0.76 (0.26-2.19) P =.70 |
| EINSTEIN34 (DVT/PE) | Rivaroxaban | VTE | Weight ≥120 kg | 1 | 303/8271 (3.7%) | 1.9% | 2.8% | N/A | N/A | NA | N/A |
| Study . | DOAC . | Anticoagulation indication . | Obesity definition . | Follow‐up duration, y . | n, obesity/N, total population . | Efficacy outcome . | Safety outcome . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DOAC . | VKA . | P and/or HR (95% CI) . | DOAC . | VKA . | P and/or HR (95% CI) . | ||||||
| RE-LY36 | Dabigatran | AF | BMI >36 kg/m2 | 1 | 1811/18113 (10%) | 0.9%* | 1.3% | P = .6 | 4.4%* | 3.7% | P = .55 |
| 1.2%† | 3%† | ||||||||||
| ARISTOTLE35 | Apixaban | AF | Weight >120 kg | 1 | 982/18139 (5.4%) | 0.44% | 1.13% | 0.39 (0.12-1.22) | 1.55% | 2.08% | 0.74 (0.37-1.50) |
| ENGAGE-AF TIMI 4825 | Edoxaban | AF | BMI ≥40 kg/m2 | 2.8 (median) | 1149/21028 (5.5%) | 2.2%§ | 1.4%§ | 1.37 (0.37-5.05)§ | 6.7%§ | 8.2%§ | 0.92 (0.54-1.57)§ |
| 3.2%ǁ | 1.4%ǁ | 2.01 (0.58-6.97)ǁ | 4.1%ǁ | 8.2%ǁ | 0.47 (0.26-0.85)ǁ | ||||||
| EINSTEIN34 (DVT/PE) | Rivaroxaban | VTE | Weight ≥100 kg | 1 | 1393/8271 (16.8%) | 2.3% | 2.0% | 1.12 (0.55-2.30) P = .25 | 0.9% | 1.2% | 0.76 (0.26-2.19) P =.70 |
| EINSTEIN34 (DVT/PE) | Rivaroxaban | VTE | Weight ≥120 kg | 1 | 303/8271 (3.7%) | 1.9% | 2.8% | N/A | N/A | NA | N/A |
HR, hazard ratio. See Table 1 for expansion of other abbreviations.
Dabigatran 150 mg twice daily.
Dabigatran 110 mg twice daily.
Edoxaban 60 mg daily.
Edoxaban 30 mg daily.
In addition to RCTs, data from a large prospective registry provides additional evidence. The Dresden NOAC Registry enrolled 3432 patients on dabigatran, rivaroxaban, apixaban, or edoxaban for either AF (69.3%) or VTE (30.7%), and 31.4% (N = 1077) of patients had BMI ≥30 kg/m2. When compared with normal weight patients (BMI <25 kg/m2), overweight (BMI 25-29.9 kg/m2) and obese (BMI ≥30 kg/m2) patients had lower rates of all clinical outcomes (cardiovascular, major bleeding, and all-cause mortality), a phenomenon known as “obesity paradox.”37 The number of patients in the morbidly obese category (BMI >40 kg/m2) remained quite small (N = 98) in this registry, with a combined effectiveness end point (stroke, transient ischemic attack, systemic embolism, or VTE) of 0.49 in 100 (95% confidence interval [CI] 0.01-2.71) patient-years as compared with the rate in the overall study population of 3.3 in 100 patient-years.37
Retrospective studies
Seven recent retrospective studies have tried to address the knowledge gap of DOAC use in the morbidly obese and are summarized in Tables 3 and 4.38-44 Five are smaller retrospective studies,38,41-44 whereas the larger 2 use the International Classification of Diseases (ICD) codes from the administrative database.39,40 These studies vary in design, with 3 comparing groups with different weight/BMI cutoffs (Table 3),38-40 and the other 4 comparing outcomes with a DOAC vs warfarin in the morbidly obese population (weight >120 kg and/or BMI >40 kg/m2) (Table 4).41-43 Two studies include VTE patients only,38,39 1 study AF patients only,41 whereas the rest include both VTE and AF.40,42-44 For thrombosis outcomes, all studies showed no significant differences when comparing between weight/BMI groups, or comparing between DOAC and warfarin groups. As for major bleeding events, most studies showed a numerically increased risk of major bleeding with warfarin and in lower weight/BMI groups, although none reached statistical significance. Given the nature of retrospective studies, patients were not randomized to selected anticoagulants, and often the comparison groups had obvious differences in baseline characteristics, so the risk for confounding was high. For example, Kushnir et al found that patients receiving warfarin were significantly older, had higher Charlson comorbidity and CHA2DS2-VASc scores, and a higher percentage had very high BMI of ≥50 kg/m2.42 In the study by Perales et al, chronic kidney and liver disease were significantly more common in the warfarin group.44 This indicates that clinicians favored warfarin in the perceived “high-risk” population, likely due to their familiarity with warfarin over DOACs in this population. Only 2 studies (Kido et al41 and Kushnir et al42 ) have reported results adjusted for confounders. Even in the studies where adjusted analyses were attempted, residual confounding was likely and could have influenced results. Therefore, although these retrospective studies added to the current literature and revealed results similar to the subgroup analyses from prospective studies, their results should be interpreted with caution.
Summary of retrospective studies, comparing between different weight groups in patients on DOACs
| Study/Year . | N . | Anticoagulant . | Anticoagulation indications . | Wright group . | Efficacy outcome: thrombotic events . | Safety outcome: major or overt bleeding, % . | |
|---|---|---|---|---|---|---|---|
| Arachchillage et al/201638 | 167 | R | VTE* | >120 kg (n = 44) | 2.3% | 0 | |
| ≤120 kg (n = 123) | 3.3% | 1.6 | |||||
| Aloi et al/201939 | 1196 | D, R, A | VTE | ≥120 kg (n = 133) | 0.8% | OR, 0.66; 95% CI, 0.09-5.14; P = .69 | N/A |
| <120 kg (n = 1063) | 1.1% | ||||||
| Netley et al/201940 | 3458 | D, R, A | VTE and AF | BMI > 40 kg/m2 (n = 595) | 1.5% | 1.2 | |
| BMI ≤ 40 kg/m2 (n = 2863) | 1.2% | 2.2 | |||||
| Study/Year . | N . | Anticoagulant . | Anticoagulation indications . | Wright group . | Efficacy outcome: thrombotic events . | Safety outcome: major or overt bleeding, % . | |
|---|---|---|---|---|---|---|---|
| Arachchillage et al/201638 | 167 | R | VTE* | >120 kg (n = 44) | 2.3% | 0 | |
| ≤120 kg (n = 123) | 3.3% | 1.6 | |||||
| Aloi et al/201939 | 1196 | D, R, A | VTE | ≥120 kg (n = 133) | 0.8% | OR, 0.66; 95% CI, 0.09-5.14; P = .69 | N/A |
| <120 kg (n = 1063) | 1.1% | ||||||
| Netley et al/201940 | 3458 | D, R, A | VTE and AF | BMI > 40 kg/m2 (n = 595) | 1.5% | 1.2 | |
| BMI ≤ 40 kg/m2 (n = 2863) | 1.2% | 2.2 | |||||
A, apixaban; D, dabigatran; OR, odds ratio; R, rivaroxaban. See Table 1 for expansion of other abbreviations.
Median follow-up, 14 months.
Summary of retrospective studies, comparing DOACs vs warfarin in obese patients
| Study/Year . | N . | Inclusion criteria . | Anticoagulants . | Efficacy outcome: thrombotic events . | Safety outcome: major bleeding events . | ||
|---|---|---|---|---|---|---|---|
| Kido et al/201941 | 128 | AF, BMI > 40 kg/m2 or weight > 120 kg | DOAC (D,R,A) (n = 64) | 1.75%/y | RR, 0.81; 95% CI, 0.20-3.27; P = .77* | 2.18%/y | RR, 0.37; 95% CI, 0.12-1.15; P = .09* |
| Warfarin (n = 64) | 2.07%/y | 4.97%/y | |||||
| Kushnir et al/201942 | 429 | AF, BMI > 40 kg/m2 | DOAC (R,A) (n = 277) | 1.8% | P = 1.00 | 2.9% | OR, 0.43; 95% CI, 0.16-1.13; P = .087* |
| Warfarin (n = 152) | 1.3% | 7.9% | |||||
| Kushnir et al/201942 | 366 | VTE, BMI > 40 kg/m2 | DOAC (R,A) (n = 199) | 2.0% | P = .69 | 1.5% | P = .60† |
| Warfarin (n = 167) | 1.2% | 2.4% | |||||
| Kalani et al/201943 | 180 | VTE and AF, BMI ≥ 40 kg/m2 and/or weight ≥ 120 kg | DOAC (D,R,A) (n = 90) | 12.2% | OR, 1.11; 95% CI, 0.45-2.78; P = .82 | 2.2% | OR, 0.66; 95% CI, 0.11-4.04; P = .65 |
| Warfarin (n = 90) | 11.1% | 3.3% | |||||
| Perales et al/201944 | 176 | VTE and AF, BMI > 40 kg/m2 or weight > 120 kg | DOAC (R) (n = 84) | 2.4% | P = .68 | 8.3%‡ | P = .06 |
| Warfarin (n = 92) | 4.3% | 2.2%‡ | |||||
| Study/Year . | N . | Inclusion criteria . | Anticoagulants . | Efficacy outcome: thrombotic events . | Safety outcome: major bleeding events . | ||
|---|---|---|---|---|---|---|---|
| Kido et al/201941 | 128 | AF, BMI > 40 kg/m2 or weight > 120 kg | DOAC (D,R,A) (n = 64) | 1.75%/y | RR, 0.81; 95% CI, 0.20-3.27; P = .77* | 2.18%/y | RR, 0.37; 95% CI, 0.12-1.15; P = .09* |
| Warfarin (n = 64) | 2.07%/y | 4.97%/y | |||||
| Kushnir et al/201942 | 429 | AF, BMI > 40 kg/m2 | DOAC (R,A) (n = 277) | 1.8% | P = 1.00 | 2.9% | OR, 0.43; 95% CI, 0.16-1.13; P = .087* |
| Warfarin (n = 152) | 1.3% | 7.9% | |||||
| Kushnir et al/201942 | 366 | VTE, BMI > 40 kg/m2 | DOAC (R,A) (n = 199) | 2.0% | P = .69 | 1.5% | P = .60† |
| Warfarin (n = 167) | 1.2% | 2.4% | |||||
| Kalani et al/201943 | 180 | VTE and AF, BMI ≥ 40 kg/m2 and/or weight ≥ 120 kg | DOAC (D,R,A) (n = 90) | 12.2% | OR, 1.11; 95% CI, 0.45-2.78; P = .82 | 2.2% | OR, 0.66; 95% CI, 0.11-4.04; P = .65 |
| Warfarin (n = 90) | 11.1% | 3.3% | |||||
| Perales et al/201944 | 176 | VTE and AF, BMI > 40 kg/m2 or weight > 120 kg | DOAC (R) (n = 84) | 2.4% | P = .68 | 8.3%‡ | P = .06 |
| Warfarin (n = 92) | 4.3% | 2.2%‡ | |||||
The values are after controlling for confounders.
P value was obtained from time to major bleeding analysis.
The safety outcomes include major bleeding and clinically relevant nonmajor bleeding, 1-year follow-up in this study.
Using large health care claims database to identify morbidly obese patients by ICD-9 codes, 2 studies have shown that the risks of thrombosis and major bleeding were comparable in patients receiving rivaroxaban vs warfarin for either AF or VTE.45,46 The utilization of health care resources, including hospitalization rate, length of stay, and outpatient visits were significantly lower with rivaroxaban compared with warfarin.44-46
Taken together, all of these studies provide evidence that even in patients with morbid obesity (weight > 120 kg and/or BMI >40 kg/m2), there are no differences in the risks of thrombosis or major bleeding between DOACs and VKA. More data are needed, especially in the VTE population. However, given the mounting evidence for the efficacy and safety of DOACs in the morbidly obese population since the publication of the ISTH guidance, the cutoff of weight of 120 kg and/or BMI of 40 kg/m2 as a limit for DOACs should be reevaluated.
Treatment recommendations for case 1
After extensive discussion of risks and benefits of different anticoagulant options, as well as the evidence regarding the use of DOACs in morbidly obese patients, the patient preferred a DOAC over warfarin. He was started on standard-dose rivaroxaban for his acute DVT. At 3-month follow-up, he was doing well with resolution of symptoms from the DVT and no evidence of bleeding.
Treatment recommendations for case 2
A similar discussion was carried out with this patient, and she also preferred a DOAC. She was discharged with apixaban 5 mg twice daily for stroke prevention and was doing well without evidence of thromboembolic events or bleeding at 6-month follow-up.
Case 3: anticoagulation in a patient after bariatric surgery
A 49-year-old woman presented with chest pain and shortness of breath 10 days after a spine fusion surgery and was found to have an acute PE on computed tomography (CT) scan. The patient had a history of morbid obesity and underwent sleeve gastrectomy 5 years ago. She had lost 50 kg since the surgery. Her current weight is 121 kg with BMI of 39 kg/m2. What anticoagulant should be recommended for her?
Evidence for anticoagulant use after bariatric surgery
In the past decade, bariatric surgery has become 1 of the main treatment options for morbid obesity and has demonstrated significant efficacy in achieving substantial weight loss. Over 200 000 bariatric procedures are performed annually in the United States and this number continues to rise.47 Given obesity, surgery, and other concurrent risk factors such as immobilization, this patient population has a high risk of VTE. There is little evidence on the efficacy and safety of DOACs in patients who have had bariatric surgery. Different types of bariatric surgeries lead to various effects on drug absorption, depending on the site of bypass and the main sites of absorption for individual DOACs. For example, sleeve gastrectomy bypasses the stomach whereas Roux-en-Y gastric bypass surgery circumvents the duodenum and proximal jejunum. Therefore, the absorption of rivaroxaban, which is mainly in the stomach and proximal small intestine, is expected to be affected more in patients with sleeve gastrectomy, whereas apixaban might not be affected as much as it is absorbed throughout the gastrointestinal tract, with the distal small intestine and ascending colon accounting for 55% of its absorption.15,48
To investigate the effect of bariatric surgery on rivaroxaban, Kröll et al gave a single 10-mg dose of rivaroxaban to 12 patients undergoing bariatric surgery (6 Roux-en-Y gastric bypass and 6 sleeve gastrectomy) at 3 time points: prior to, 3 days after, and 6 to 8 months after surgery.49,50 They showed that the PK profiles were comparable at all time points and concluded that PK of prophylactic dose of rivaroxaban was not affected by bariatric surgery. However, another study measured peak DOAC levels in 18 patients after bariatric surgery (a median of 4.9 years after surgery) and 18 matched controls.51 They found that 5 of 18 patients (27.8%) in the postsurgery group had a lower than expected peak level, compared with none (0%) in the control group. Interestingly, all 5 patients with a low level were on rivaroxaban and had sleeve gastrectomy or banding. This study raised concerns for decreased rivaroxaban absorption in patients where a significant portion of stomach was bypassed. In addition, the therapeutic doses of rivaroxaban (15 mg and 20 mg) are known to depend on food for absorption. Restriction of caloric intake after gastrectomy could have a greater interference with the absorption of the therapeutic dose of rivaroxaban than with the prophylactic dose.52 More studies are needed in this population, and clinical trials are ongoing to evaluate the PK and PD of apixaban before and after bariatric surgery (NCT02406885, NCT03448783).
Give the rationale for altered absorption of DOACs in patients after bariatric surgery, limited evidence supporting that this might be true, and lack of studies reporting clinical outcomes in this population, we avoid DOAC use after bariatric surgeries. Use of either VKA where INR can be monitored or LMWH that avoids the gastrointestinal system remains our preferred choice in this population.
Treatment recommendations for case 3
An extensive discussion was had with the patient about the limited evidence in the use of DOACs after bariatric surgery. The patient agreed with warfarin treatment. She completed 3 months of anticoagulation without bleeding events for her provoked PE and stopped anticoagulation. She had no evidence of VTE recurrence at 1 year.
Case 4: anticoagulation as VTE prophylaxis in morbidly obese patients
A 60-year-old man presents for a total hip replacement for chronic osteoarthritis of the right knee. His medical history includes obesity with weight of 130 kg and BMI of 45 kg/m2, hypertension, hyperlipidemia, and a history of a postoperative DVT 3 years ago after left knee replacement. His orthopedic surgeon asks for recommendations for perioperative thromboprophylaxis.
Evidence for use of VTE prophylaxis in the obese
Obesity is a risk factor for osteoarthritis and the need of joint replacement, and effective thromboprophylaxis, is essential in the prevention of surgery-related VTE.53 Large RCTs have established the role of various DOACs as thromboprophylaxis for patients undergoing total hip or knee replacement.54 The effects of BMI in this population have been evaluated in several post hoc analyses. The RE-MODEL and RE-NOVATE I and II studies compared dabigatran to enoxaparin after hip or knee replacement.55 A total of 5686 patients were enrolled in all 3 studies combined and, of these, 1826 (32.1%) had a BMI >30 kg/m2 with 104 (1.8%) having a BMI >40 kg/m2. A post hoc analysis revealed that there were no differences in the rate of VTE or major hemorrhage between dabigatran and enoxaparin in the group with BMI >30 kg/m2.55 Similarly, the RECORD trials compared rivaroxaban with enoxaparin as thromboprophylaxis after total hip and knee replacement.56 Among the total of 12 355 patients enrolled in all 4 RECORD trials, 3.6% (N = 445) had BMI ≥40 kg/m2. The adjusted analysis showed that there were no significant differences in the incidence of VTE or bleeding events in patients with BMI ≥40 kg/m2 compared with those with BMI <40 kg/m2. In the ADVANCE trials, apixaban was compared with enoxaparin for thromboprophylaxis after hip or knee arthroplasty. A pooled analysis of the ADVANCE-2 and -3 studies included 3065 patients (36.2%) with a BMI ≥30 kg/m2.57 BMI did not change the treatment effects of apixaban vs enoxaparin. A meta-analysis including studies involving apixaban and dabigatran in postarthroplasty patients again demonstrated that the efficacy and safety of DOACs were comparable to enoxaparin for VTE prevention in overweight and obese patients.58 However, apixaban showed a superior efficacy and low-dose dabigatran (150 mg as compared with the standard 220 mg) showed a reduced efficacy compared with enoxaparin in the obese population (BMI ≥30 kg/m2) but not overweight population (BMI 25-30 kg/m2). The authors called for action to find the optimal dose for each DOAC as thromboprophylaxis in the obese population undergoing arthroplasty.
The available evidence supports the use of a standard prophylactic dose of DOACs in the obese, same as nonobese, patients, although most data involve the “less” obese category with BMI cutoff of 30 kg/m2. The analysis of RECORD trials focusing on BMI cutoff of 40 kg/m2 is instrumental and results are reassuring. Based on the literature cited above, we would treat the obese and morbidly obese population with the standard dose of DOACs for VTE prophylaxis.
Treatment recommendations for case 4
This patient can be given the standard prophylactic dose of either dabigatran, rivaroxaban, or apixaban for 10 to 14 days after his knee replacement.
Conclusions and future perspectives
Table 5 lists our summary recommendations for the use of DOACs in obese patients in these specific situations. Despite the concern for altered PK and anticoagulant effects of DOACs in the obese population, available literature has not shown evidence of inferior efficacy or safety with DOACs in the obese. In patients with a weight <120 kg or BMI <40 kg/m2, data from large RCTs were reassuring for the efficacy and safety of DOACs and we use them as in nonobese adults. On the other hand, evidence of DOAC use in the morbidly obese population (weight ≥120 kg and/or BMI ≥40 kg/m2) remains limited (although accumulating), particularly in the VTE population, and there are no RCTs to date. Future studies should focus on evaluating the efficacy and safety of DOACs in the morbidly obese population, especially with VTE. Until then, an informed discussion should be carried out with patients to allow individualized decision-making.
Summary statements
| Summary statements . |
|---|
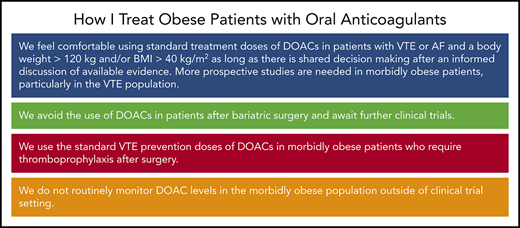
| 1. We feel comfortable using standard treatment doses of DOACs in patients with VTE or AF and a body weight >120 kg and/or BMI >40 kg/m2 as long as there is shared decision-making after an informed discussion of available evidence; More prospective studies are needed in morbidly obese patients, particularly in the VTE population |
| 2. We avoid the use of DOACs in patients after bariatric surgery and await further clinical trials |
| 3. We use the standard VTE prevention doses of DOACs in morbidly obese patients who require thromboprophylaxis after surgery |
| 4. We do not routinely monitor DOAC levels in the morbidly obese population outside of clinical trial setting |
| Summary statements . |
|---|
| 1. We feel comfortable using standard treatment doses of DOACs in patients with VTE or AF and a body weight >120 kg and/or BMI >40 kg/m2 as long as there is shared decision-making after an informed discussion of available evidence; More prospective studies are needed in morbidly obese patients, particularly in the VTE population |
| 2. We avoid the use of DOACs in patients after bariatric surgery and await further clinical trials |
| 3. We use the standard VTE prevention doses of DOACs in morbidly obese patients who require thromboprophylaxis after surgery |
| 4. We do not routinely monitor DOAC levels in the morbidly obese population outside of clinical trial setting |
Another important consideration is that different DOAC agents could have different PK profiles and should be investigated individually. This was already seen in the limited existing data. Measurement of DOAC levels has been recommended by the international guidance statement, but the appropriate “on-therapy” range in the obese population and correlation of drug levels with clinical outcomes are both unknown. Therefore, currently we do not routinely monitor DOAC levels in the obese outside of clinical trial setting. Dedicated studies in obese and morbidly obese patients to address this knowledge gap are needed to determine the optimal care of these patients.
Acknowledgment
The authors thank Kerry A. Rogers for reviewing and commenting on the manuscript.
Authorship
Contribution: T.-F.W. and M.C. both contributed to study concept and design, acquisition, analysis, and interpretation of data, and drafting and revision of the manuscript.
Conflict-of-interest disclosure: M.C. reports research grants from Pfizer, Bristol-Myers Squibb, and Leo Pharma, and consultancy honoraria from Pfizer, Bayer, Sanofi, Servier, and Leo Pharma. T.-F.W. reports no competing financial interests.
Correspondence: Tzu-Fei Wang, The Ohio State University Wexner Medical Center, A332B Starling Loving Hall, 320 West 10th Avenue, Columbus, OH 43210; e-mail: tzu-fei.wang@osumc.edu.