Abstract
Administering asparaginase has always been problematic in adults because most general oncologists who treat adults are not familiar with its usage and toxicity. The toxicity profile of the drug is unique and is not observed with any other chemotherapy agent. Furthermore, asparaginase is almost exclusively used in acute lymphoblastic leukemia (ALL), which is a very rare cancer in adults. Currently, the long-acting pegylated form (pegasparaginase) is the only Escherichia coli–derived asparaginase available in the United States. The use of pediatric regimens is likely to lead to more adult patients receiving multiple doses of pegasparaginase. However, oncologists who treat adults may be reluctant to use pegasparaginase or may unnecessarily discontinue administering it because of certain adverse effects. As a result, the clinical benefit of multiple doses of pegasparaginase will be missed. Despite the fact that pegasparaginase is associated with unique toxicities, the majority are nonfatal, manageable, and reversible. Here, we describe real-life cases of adults with ALL who were treated with pediatric-inspired regimens that incorporated pegasparaginase to illustrate the management of several pegasparaginase-associated adverse effects and guide whether and how to continue the drug.
Introduction
Acute lymphoblastic leukemia (ALL) in adults is associated with inferior survival outcomes when compared with ALL in children, which is primarily because of the considerable risk of relapse in adults.1-6 Although increased risk of relapse is partly related to the finding that adults more frequently develop ALL with unfavorable genetics,7 it is also attributed to inadequate chemotherapeutic regimens historically used to treat adults.
Retrospective comparisons of adolescents and young adults with ALL have consistently demonstrated significantly longer survival for patients who are treated by pediatricians with a pediatric regimen than patients of the same age treated by oncologists who treat adults using adult regimens.8-11 This prompted extension of pediatric or pediatric-inspired regimens to adults with ALL, with upper ages ranging from 39 to 60 years.12-18 These studies have confirmed the feasibility and safety of administering such regimens to adults with ALL and have improved outcomes compared with historical adult regimens.12-18 A key element of pediatric regimens for ALL is incorporation of multiple doses of postremission asparaginase, a bacterial enzyme that hydrolyses serum asparagine into aspartic acid and ammonia, thus depleting serum asparagine and depriving ALL cells of this nutrient.
Several randomized pediatric studies reported the favorable impact of intensive postremission asparaginase on leukemia outcomes.19-21 For example, the Dana-Farber Consortium 77-01 study reported that patients treated with intensive postremission high-dose asparaginase had significantly superior outcomes compared with patients who did not receive postremission asparaginase.19 Likewise, the POG 8704 study in T-cell ALL reported an improved 4-year continuous complete remission (CR) rate for patients randomly assigned to postremission high-dose asparaginase vs no asparaginase consolidation (68% vs 55%).21 The CCG 1882 study reported superior event-free survival in high-risk children who received 5 cycles of postremission asparaginase compared with those who received 1 cycle (75% vs 55%).20 Because of the success of adding asparaginase to pediatric regimens for ALL, recent prospective adult studies have incorporated more doses of asparaginase compared with historical adult regimens (6 to 15 vs 0 to 2 cycles).12,14,22-26 Administering asparaginase can be problematic in adults because general oncologists who treat adults may not be familiar with its usage and toxicity. The toxicity profile of asparaginase, summarized in Table 1, is unique and is not observed with other chemotherapy agents. Furthermore, asparaginase is almost exclusively used in ALL, which is a rare cancer in adults.
The rate and risk factors for pegasparaginase toxicities in adults
| Toxicity . | Any grade (%) . | High grade (≥ 3) (%) . | Risk factors . |
|---|---|---|---|
| Hypersensitivity | 7-22 | 4-10 | Second dose and future doses, HLA-DRB1*07:01 polymorphism, no concurrent rituximab administration, younger age, no pre-medications |
| Hyperbilirubinemia | 86 | 24-39 | During the induction cycle, older age, obesity, higher dose of pegasparaginase, low albumin, low platelet count, CC genotype of rs4880 polymorphism |
| Pancreatitis | 24 | 5-13 | Older age, high-risk ALL stratification, germline polymorphisms in ULK2 variant rs281366 and RGS6 variant rs17179470 |
| Hypertriglyceridemia | 77 | 11-51 | Beyond first cycle, high BMI, younger age |
| Thrombosis | 11-27 | First cycle, older age, obesity, mediastinal mass, cryoprecipitate replacement | |
| Hypofibrinogenemia (<100) | 48-51 | First cycle, severe obesity (BMI >35) | |
| Hyperglycemia | 91 | 31-33 | Concomitant use of steroid |
| Toxicity . | Any grade (%) . | High grade (≥ 3) (%) . | Risk factors . |
|---|---|---|---|
| Hypersensitivity | 7-22 | 4-10 | Second dose and future doses, HLA-DRB1*07:01 polymorphism, no concurrent rituximab administration, younger age, no pre-medications |
| Hyperbilirubinemia | 86 | 24-39 | During the induction cycle, older age, obesity, higher dose of pegasparaginase, low albumin, low platelet count, CC genotype of rs4880 polymorphism |
| Pancreatitis | 24 | 5-13 | Older age, high-risk ALL stratification, germline polymorphisms in ULK2 variant rs281366 and RGS6 variant rs17179470 |
| Hypertriglyceridemia | 77 | 11-51 | Beyond first cycle, high BMI, younger age |
| Thrombosis | 11-27 | First cycle, older age, obesity, mediastinal mass, cryoprecipitate replacement | |
| Hypofibrinogenemia (<100) | 48-51 | First cycle, severe obesity (BMI >35) | |
| Hyperglycemia | 91 | 31-33 | Concomitant use of steroid |
BMI, body mass index.
In the United States, the long-acting pegylated form (pegasparaginase) is the only available Escherichia coli–derived asparaginase. Our standard dose of pegasparaginase is 2000 IU/m2 for adults up to age 55 years compared with 2500 IU/m2 used in children because this dose seems to be safer, with adequate pharmacokinetic and pharmacodynamic properties.25-28 Of note, other groups used a lower pegasparaginase dose (1000 IU/m2) to reduce toxicities while maintaining adequate activity,24,29 with a minimum of 2 weeks of asparagine depletion.30 The NOPHO ALL2008 study in children used 1000 IU/m2 pegasparaginase and reported excellent long-term outcomes with favorable toxicity.24 The UKALL14 adult trial included 2 doses of 1000 IU/m2 pegasparaginase during induction and observed excessive induction mortality. An amendment that omitted the first dose resulted in significantly fewer deaths at induction.29 Nonetheless, limited data preclude a definitive answer on whether lowering the dose of pegasparaginase mitigates toxicities besides hepatotoxicity.26 In our practice, a dose of 1000 IU/m2 pegasparaginase is given to morbidly obese patients who have a high risk for serious liver toxicity. Other practitioners cap the dose at 3750 IU (equivalent to one vial) as a potential safety measure in patients with large body surface area to prevent excessive toxicity. Pegasparaginase is given concomitantly with other antileukemia drugs, including tyrosine kinase inhibitors as indicated. As a general rule, we recommend being faithful to the specific regimen adopted without modifications.
Recent publications on the favorable outcome of pediatric regimens (for example, the US CALGB 10403 trial12 ) will likely lead to more adult patients receiving multiple doses of pegasparaginase. However, oncologists who treat adults may still be reluctant to use pegasparaginase or may unnecessarily discontinue its administration because of adverse effects. As a result, clinical benefit of multiple pegasparaginase doses will be missed.
In 2011, an expert panel made recommendations for preventing and managing asparaginase- and pegasparaginase-associated toxicities in adults and older adolescents.31 Because we and others have increasing experience with pegasparaginase in adults, revisiting how we approach this clinical problem is timely. The following cases illustrate how several adverse effects associated with pegasparaginase have been managed and guide whether and how to continue the drug.
Case 1: pegasparaginase-induced allergic reaction
Patient 1 is a 26-year-old female diagnosed with B-cell ALL with 46,X,t(X;14)(p22;q32), immunoglobulin H translocation, and NRAS mutation who was treated by using the CALGB 10403 regimen12 and achieved a CR with persistent minimal residual disease (MRD) after induction. During consolidation, and after the third dose of intravenous pegasparaginase, she developed hypotension, swollen eyelids, chest pain, and urticarial rash. Infusion was stopped, and she received hydrocortisone, diphenhydramine, and intravenous fluids; her symptoms then resolved. She achieved MRD-negative CR.
Asparaginase is a bacterial-derived enzyme and thus can elicit an immune response manifesting as an allergic reaction, including anaphylaxis. The rate of allergic reactions in adults receiving pegasparaginase is 7% to 22%14,18,25,32 (4% to 10% of patients have grade ≥3 reactions).12,14,32 Pegasparaginase-induced allergic reactions usually occur early during the treatment course, typically with the second or third dose.12,32 After an allergic reaction, asparaginase is also inactivated likely from asparaginase-induced antibodies, which may result in re-treatment failure.33 Fortunately, after an allergic reaction to E coli–derived pegasparaginase, activity can be maintained by switching to Erwinia-derived asparaginase that has only limited cross-reactivity with E coli–derived asparaginase.34
Pegasparaginase-induced allergic reaction seems to be less common in adults than in children, but this may be a result of the use of prophylactic hydrocortisone and antihistamine pre-asparaginase in adults. Amending the adult CALGB 10403 protocol to incorporate premedication lowered the high-grade hypersensitivity reaction rate from 10% to 4%.12 In a retrospective pediatric study, premedication resulted in reduced asparaginase-induced hypersensitivity, without excessive rates of asparaginase inactivation or substitution to Erwinia-derived asparaginase.35 Therefore, in many institutions and in ongoing trials, it has become a standard practice to administer premedication to adults before each pegasparaginase dose. In a genome-wide study, patients carrying HLA-DRB1*07:01 alleles had higher incidence of allergic reactions and anti-asparaginase antibodies.36 Interestingly, there were fewer asparaginase-related allergic reactions and less switching to Erwinia-derived asparaginase in adults who were randomly assigned to rituximab in the GRAALL-2005/R study,37 perhaps because of rituximab-induced B-cell depletion and reduction of allo-immunization toward asparaginase. In children, the rate of high-grade allergic reaction was lower after intravenous pegasparaginase than after using the intramuscular route, with potentially less antibody formation.38
Neutralizing antibodies and asparaginase inactivation may occur without clinical manifestations of hypersensitivity, also called “silent inactivation,” which seems to be uncommon at a rate of less than 10%.35,39,40
The efficacy of asparaginase depends on maintaining adequate and prolonged depletion of serum asparagine. Inadequate dosing of asparaginase correlated with poor leukemia-related outcomes,39,41 and therapeutic drug monitoring (TDM) is mostly relevant in the context of hypersensitivity.40,42 Asparaginase activity can be measured using commercially available reagents. However, determining the minimal activity level that correlates with complete serum asparagine depletion is debatable, with reports ranging between 0.02 and 0.2 IU/mL serum asparagine.27,39,42,43 A detailed discussion on the relationship between drug activity level and asparagine depletion is found in the recent consensus recommendations.42 After administering pegasparaginase, the goal is to maintain therapeutic levels of asparaginase activity for 14 days or longer. Anti-asparaginase antibodies cannot be measured using commercially available reagents, and this is not a reliable technique for predicting drug inactivation because of low specificity.40 The importance of monitoring asparaginase activity levels without clinical hypersensitivity emerges from the potential to switch to Erwinia-derived asparaginase. One pediatric study showed that detecting silent hypersensitivity by monitoring enzymatic activity of asparaginase and switching to Erwinia-derived asparaginase improved overall survival compared with those who had silent inactivation but were not switched.39 Erwinia-derived asparaginase is recommended in favor of pegasparaginase for patients with clinical or silent hypersensitivity to maximize the clinical efficacy for pediatric regimens. Therefore, we suggest measuring enzymatic activity between days 3 and 7 after each dose. Several recently published algorithms have added specific details on measuring asparaginase activity.44,45
It is occasionally difficult to distinguish clinically between a mild allergic reaction to asparaginase and an infusion reaction resulting from a non-antibody–mediated process because of the rapid rise in ammonia levels post-asparaginase or preexisting anti-PEG antibodies, 33,46 which may occur after the first dose. Infusion reactions may not affect drug clearance and activity, and switching to Erwinia-derived asparaginase can be avoided. The diagnosis of allergy would be confirmed if the TDM around day 7 showed inadequate asparaginase activity.
Although premedication reduces the risk of clinical hypersensitivity, it might mask silent inactivation.35 Although TDM is not universal, we recently implemented an approach to TDM when administering pegasparaginase intravenously with routine premedication to possibly avoid unmasking silent inactivation. We switched to Erwinia-derived asparaginase for clear clinical hypersensitivity such as anaphylaxis or urticaria. For mild reactions, when it is difficult to clinically distinguish between allergy and an infusion reaction, we rely on TDM to decide whether to switch to Erwinia-derived asparaginase. We use an asparaginase activity of 0.1 IU/mL as a level associated with complete asparagine depletion,47 supported by key studies that used this level as a cutoff to switch to Erwinia-derived asparaginase.39,48 Each dose of pegasparaginase that is not given plus the dose that caused hypersensitivity or silent inactivation is substituted by 6 doses of Erwinia-derived asparaginase over 2 weeks.48 Recently, Erwinia-derived asparaginase has intermittently been in short supply and switching to provide the entire planned course of asparaginase may not possible. Clinicians have taken several approaches, often on a case-by-case basis, but without data to support any strategy. Before switching, it is critical to ensure by TDM that the reaction is bona fide hypersensitivity and not an infusion reaction.
Patient 1's diagnosis of an allergic reaction was confirmed by asparaginase activity of <0.1 IU/mL on day 7 post-dosing, and all subsequent doses of pegasparaginase were replaced with Erwinia asparaginase. She tolerated Erwinia asparaginase well with no allergic reaction.
Case 2: pegasparaginase-induced hepatotoxicity
Patient 2 is a 36-year-old Hispanic female with a body mass index of 30 who was diagnosed with B-cell ALL with JAK2 mutation. She was induced according to a pediatric-inspired regimen14 and received 2000 IU/m2 pegasparaginase on day 15. Total bilirubin started to increase 1 week post-pegasparaginase and peaked 2 weeks later at 22 mg/dL (grade 4 toxicity). After 2 weeks, the bilirubin gradually declined spontaneously to grade 1. She achieved CR with persistent MRD.
Hepatotoxicity is the most common adverse effect of pegasparaginase in adults, manifesting as hyperbilirubinemia and/or transaminitis. Although uncommon in children,49 high-grade hyperbilirubinemia (grades 3 to 4) has been reported in 24% to 39% of adults treated with pediatric regimens.12,14,18,26,29,32 The rate of transaminitis is even higher in adults at rates of 93% for any grade and ∼50% for high grade.12,32
The etiology of pegasparaginase-induced hepatotoxicity remains unknown, but it has several typical characteristics.50 The toxicity is reversible, almost always unassociated with clinical liver disease, and rarely leads to liver failure. Pegasparaginase-induced hyperbilirubinemia is mostly seen during induction, with the incidence usually declining or not recurring in subsequent cycles.12,32,50 The median duration from the time of administration of pegasparaginase until onset of high-grade hyperbilirubinemia is approximately 2 weeks, whereas the median time until toxicity recovery to grade 1 is often long and in some cases >4 weeks from the dose.26,50 Among patients who experienced high-grade hyperbilirubinemia and were re-challenged with pegasparaginase, only 18% of all subsequent doses resulted in the development of high-grade hyperbilirubenemia.50,51 In a recent small study, high-grade hyperbilirubinemia occurred only after the first dose and almost never recurred.52
The long duration of pegasparaginase-induced high-grade hyperbilirubinemia can delay subsequent cycles, but whether this has a negative impact on outcome in adults has not yet been well studied.26 Increased liver enzymes have less detrimental impact on treatment schedule because mild-to-moderate transaminitis does not dictate holding therapy or delaying cycles.
Risk of pegasparaginase-induced high-grade hyperbilirubinemia is linked to older age, obesity, low albumin, low platelet count, and administration of high doses of pegasparaginase.26,29,32,51,53,54 A specific polymorphism in the SOD2 gene (CC genotype of rs4880), a key mitochondrial enzyme that protects cells against reactive oxygen species, was associated with increased hepatotoxicity after asparaginase therapy in 1 study.55 Hepatic steatosis is often documented in patients treated with pegasparaginase, even pretreatment,51,53,56,57 but with little evidence of liver failure. Therefore, our routine practice is not to reduce the pegasparaginase dose for liver steatosis and not to perform routine pretreatment liver ultrasound.
Because hepatotoxicity is more frequent in adults and often manifests as a dramatic increase in bilirubin and liver enzymes, oncologists who treat adults may refrain from administering subsequent pegasparaginase doses. Because pegasparaginase is a key component of contemporary ALL regimens, this practice should be avoided because it may compromise the regimen efficacy, especially considering that hepatotoxicity is usually encountered early during induction therapy. The fact that hepatotoxicity is almost always reversible and often does not recur on subsequent doses is reassuring, and we recommend continuing pegasparaginase in subsequent cycles. The rate of high-grade hepatotoxicity is low with Erwinia-derived asparaginase,34,58 but no data support switching to Erwinia-derived asparaginase for hepatotoxicity, and we do not recommend this practice.
Single-case observations and small case series reported that treatment with l-carnitine can result in rapid amelioration of asparaginase-induced hyperbilirubinemia.59-62 Although this approach was successful in an animal model with pegasparaginase-induced hepatoxicity,63 larger studies are needed to confirm and better define this effect before l-carnitine treatment can be routinely recommended.
For pegasparaginase induced high-grade hyperbilirubinemia (grades 3 to 4), we hold drugs with known hepatotoxicity (eg, azoles or echinocandins) and adjust the dose and schedule of concurrent medications or chemotherapies that are metabolized by the liver. We also delay the next chemotherapy cycle until hyperbilirubinemia resolves to grade 1 and transaminitis is grade 2 or lower. We do not hold or reduce the pegasparaginase dose or switch to a different formulation for subsequent doses of asparaginase after high-grade hepatotoxicity. We may give l-carnitine for hyperbilirubinemia hoping to accelerate the normalization of hyperbilirubinemia and avoid excessive delay in subsequent cycles, but we acknowledge that no robust data currently support this approach. Hyperbilirubinemia at time of diagnosis is often related to liver involvement by ALL. Therefore, we may cytoreduce with steroids and administer pegasparaginase after the bilirubin is close to normal.
Patient 2's consolidation treatment was delayed for 2 weeks because of high-grade hyperbilirubinemia. Subsequently, she started consolidation and received the second and third doses of pegasparaginase. Her bilirubin remained normal without delaying the treatment schedule. She achieved MRD-negative CR after consolidation.
Case 3: pegasparaginase-induced thrombosis
Patient 3 is a 41-year-old female diagnosed with T-cell ALL with large mediastinal mass who was induced according to a pediatric-inspired regimen,14 and achieved CR with MRD negativity. At the end of induction, she developed swelling of the right arm; Doppler ultrasound confirmed deep vein thrombosis (DVT). She started a therapeutic dose of enoxaparin plus platelet transfusion during periods of thrombocytopenia. We replaced anti-thrombin III (ATIII) when activity level was <50% while she was receiving anticoagulation therapy.
Asparaginase predisposes patients to thrombosis because it can reduce levels of natural anticoagulants such as protein C, protein S, plasminogen, and ATIII.64-66 In a small study, all patients developed low ATIII levels corresponding to duration of pegasparaginase activity.27 The rate of thrombosis requiring anticoagulation in adults treated with pegasparaginase is 5% to 27%,12,14,18,23,26,32,67 but the rate of thrombosis was reported as high as 34% in adults treated with l-asparaginase.68 Thrombosis is predominantly venous rather than arterial.32 It occurs more frequently during the induction cycle,32 possibly from a higher hypercoagulable state related to active leukemia, prolonged hospitalization, and the excessive use of steroids in this cycle.69 The risk of asparaginase-induced venous thromboembolism (VTE) increases with age, obesity, a mediastinal mass at diagnosis, and lower white blood cell counts at diagnosis.32,67,68 Cavernous sinus thrombosis (CST) is a noteworthy rare but serious complication reported in 1% to 3% of adults treated with asparaginase and is often accompanied by superimposed intracranial bleeding (35%).32,70 Although CST is fatal in only ∼5% of patients who develop it, the event can carry significant morbidity.70
Treatment of pegasparaginase-induced VTE (DVT and pulmonary embolism) is the same as that for general VTE management: low molecular weight heparin started promptly and continued throughout pegasparaginase treatment and for at least 3 months. Platelet transfusion may be required early in VTE to allow the delivery of therapeutic doses of anticoagulation. One study reported that re-challenging asparaginase with anticoagulation was safe and allowed most patients to receive the intended doses of asparaginase; the overall survival was similar between patients with and without VTE.68 In our experience, none of 10 patients who developed VTE and subsequently resumed pegasparaginase while receiving anticoagulation therapy had recurrent VTE.32 Heparins require adequate ATIII to function. Checking and replacing ATIII while asparaginase is active during concurrent anticoagulation is physiologically conceivable and could be applied, although it is not well supported by clinical data. We treat CST with anticoagulation therapy similar to the way we treat other acute VTEs. Anticoagulation is recommended even in cases with concomitant intracranial bleed. No strong data support the continuation of pegasparaginase after CST, and the decision may depend on the clinical severity of thrombosis in each individual patient. Because long-term neurologic consequences may occur, we recommend discontinuing pegasparaginase after CST.
Prophylactic ATIII replacement is controversial because the effect of ATIII on VTE is inconsistent in different trials. For example, ATIII replacement in the PARKAA randomized study was safe with a trend toward reducing VTE in children during l-asparaginase therapy (28% vs 37%).71 In a retrospective analysis of adults treated with pegasparaginase, ATIII replacement did not reduce VTE rate (17% vs 11%; P = .52), but it did increase the overall treatment cost.72 In contrast, the CAPELAL retrospective analysis in adults treated with l-asparaginase showed benefit with ATIII replacement on reduction of VTE risk (4.8% vs 12.2%; P = .04).73 Given these results, the small number of patients in each study, inconsistency of threshold ATIII levels for replacement, different doses of ATIII given, and the high cost of ATIII replacement, our own approach is to not supplement ATIII to prevent pegasparaginase-induced thrombosis. Larger prospective studies are needed to confirm a benefit to justify the cost of ATIII replacement for VTE prevention.
Despite the high rate of pegasparaginase-induced laboratory hypofibrinogenemia (<100 mg/dL in 48% of patients), the risk of major bleeding is low overall. In fact, we and others have observed a correlation between cryoprecipitate replacement for severe hypofibrinogenemia and onset of VTE during pegasparaginase therapy.32,74 In our experience, 35% of patients who developed VTE during pegasparaginase therapy had received cryoprecipitate replacement in the same cycle for hypofibrinogenemia.32 Given the higher risk of thrombosis, we advise caution in routinely correcting laboratory abnormalities in the absence of active bleeding.
Another question is whether to use prophylactic anticoagulation. A recent Dana-Farber Cancer Institute ALL pediatric protocol in adults used prophylactic anticoagulation and reported lower-risk VTE (41% vs 28%; P = .32) without increasing high-grade bleeding (0% vs 6%; P = .26).23 In a recent pediatric study, all patients received prophylaxis during induction, but those who received enoxaparin or activity-adapted ATIII replacement had a lower rate of VTE compared with those who received low-dose unfractionated heparin.75 However, this is still controversial, and we and others so far have not taken this approach. Nonetheless, we offer enoxaparin prophylaxis to hospitalized ALL patients with an adequate platelet count.
Treatment of patient 3 resumed with all scheduled doses of pegasparaginase while she continued to receive enoxaparin. No additional DVTs were diagnosed.
Case 4: pegasparaginase-induced hypertriglyceridemia
Patient 4 is a 44-year-old female diagnosed with B-cell ALL, normal karyotype with MLL2 mutation. She was induced with a pediatric-inspired regimen,14 achieved MRD-negative CR, and received consolidation. A routine blood check showed hypertriglyceridemia (triglyceride level peak of 3600 mg/dL; grade 4 toxicity).
Hypertriglyceridemia is a common laboratory abnormality during asparaginase therapy. Despite high triglyceride levels (in up to ∼50%),32 in our experience, it resolves spontaneously and quickly. Pegasparaginase-induced high-grade hypertriglyceridemia usually occurs after the first cycle of induction and is more frequent during consolidation cycles.12,32 In adults, we observed a direct correlation between risk of pegasparaginase-induced high-grade hypertriglyceridemia and increased body mass index, but we noted an inverse association with increased age.32
Because hypertriglyceridemia is a risk factor for pancreatitis and because both toxicities can occur post-asparaginase, clinicians may wish to treat hypertriglyceridemia to avoid pancreatitis. However, we and others have found no direct relationship between pegasparaginase-induced hypertriglyceridemia (grade or timing) and clinical pancreatitis.32,76,77
In our experience, hypertriglyceridemia does not require any medical intervention, and the occurrence of any grade should not delay or preclude administering subsequent doses of pegasparaginase. We offer gemfibrozil for high-grade hypertriglyceridemia, but any benefit of lowering triglyceride levels faster than the natural drop has not been established.
Pegasparaginase toxicities also include hyperglycemia, which often temporarily requires insulin. Ammonia is a product of asparaginase hydrolyzation of asparagine and hyperammonemia may manifest as transitory metabolic encephalopathy.78-80
Patient 4 was asymptomatic with normal serum lipase and amylase levels, and her triglyceride levels normalized within 3 weeks without intervention. Her subsequent pegasparaginase doses were also associated with asymptomatic hypertriglyceridemia that resolved without intervention. She did not experience clinical pancreatitis during therapy.
Case 5: pegasparaginase-induced pancreatitis
Patient 5 is a 22-year-old Hispanic male diagnosed with B-cell ALL with normal karyotype who was induced according to the pediatric CALGB 10403 regimen12 and achieved MRD-negative CR. While he was receiving consolidation therapy after the second pegasparaginase dose, he developed clinical pancreatitis. Imaging showed acute interstitial edematous pancreatitis and peripancreatic fluid collection without signs of necrosis or pseudocyst. He received supportive care and his symptoms resolved.
Clinical pancreatitis occurs in 5% to 14% of adults treated with pegasparaginase12,14,18,32 ; however, chemical increase in pancreatic enzymes in the absence of symptoms or imaging findings occurs more frequently (24%).32 Asparaginase-associated pancreatitis (AAP) can pose significant morbidities. Detailed analysis of AAP in adults is lacking. However, in pediatric registries for AAP, severe pancreatitis necessitating a mechanical ventilator was seen in 8%, whereas 26% to 30% of AAP patients developed pseudocyst, 25% developed necrosis, and 2% of patients died as a result.81,82 During the acute phase of AAP, 21% of children required acute insulin therapy and 11% continued to receive insulin 1 year after the incident.82
The pathophysiology and predicted clinical factors for AAP remain unknown. In a systematic review, older age, pegasparaginase formulation, and high-risk ALL stratification were associated with increased risk of AAP.83 Wolthers et al84 observed a correlation between germline polymorphisms (ULK2 variant rs281366, RGS6 variant rs17179470) and increased risk of AAP, especially in children younger than age 10 years. Such genomic studies are lacking in adults.
Development of AAP is a clear contraindication for continuing pegasparaginase because the recurrence rate with re-challenging doses is 46% to 63%, with half the cases being severe.82,85 Similarly, AAP is observed with Erwinia-derived asparaginase,34 and switching the asparaginase formulation should be avoided for patients who develop AAP with the pegylated formulation. However, when lipase or amylase are increased without clinical manifestations (ie, chemical pancreatitis), asparaginase can be continued regardless of levels.
Octreotide prophylaxis was proposed to prevent AAP, but data showing efficacy of prophylaxis are limited.86 If AAP occurs, patients benefit from early diagnosis and aggressive supportive measures, including hydration, pain control, parenteral feeding, and antimicrobial coverage. In severe cases, patients may require surgical intervention.
Pegasparaginase toxicity leading to early and permanent discontinuation (ie, because of the development of pancreatitis) can be challenging. Using a chemotherapy regimen without all prescribed asparaginase doses to treat a patient who can no longer tolerate pegasparaginase is associated with inferior outcome.87 Current data do not provide guidance for this scenario, and we recommend addressing this problem on a case-by-case basis. For example, we might recommend consolidation with allogeneic hematopoietic cell transplantation to overcome the anticipated increased risk of relapse from omitting the remaining doses of pegasparaginase. Other clinicians might resume the chemotherapy regimen without modification aside from omitting pegasparaginase or might consider intensifying consolidation with high-dose methotrexate (ie, in protocols that use the Capizzi approach). However, this remains an important issue that will require additional studies to address.
Treatment of patient 5 was subsequently resumed, but we permanently discontinued pegasparaginase. Eventually, he started maintenance therapy, but he experienced isolated central nervous system relapse 6 months later. He achieved remission with combination chemotherapy with frequent intrathecal chemotherapy.
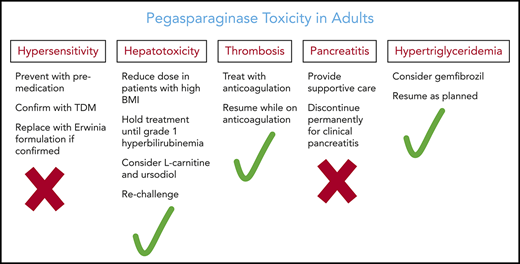
In conclusion, pegasparaginase contributes to higher cure rates in adults with ALL. Although pegasparaginase is associated with unique toxicities, the majority are nonfatal, manageable, and reversible (Table 2). Careful patient education and follow-up is essential for early detection and management of the toxicities, in most cases without dose modification. Unnecessary early discontinuation or dose reduction of pegasparaginase should be avoided and may significantly compromise efficacy, which will diminish the chances of curing the patient.
Management and prevention of pegasparaginase toxicities
| Toxicity . | Management . | Prevention . |
|---|---|---|
| Hypersensitivity | Administer corticosteroid and antihistamine | Pre-medicate with hydrocortisone and antihistamine |
| Replace future doses of l-asparaginase with Erwinia asparaginase | Infuse slowly over 2 h | |
| Hyperbilirubinemia | Adjust other medications and delay subsequent cycle until grade 1 is achieved | Avoid hepatotoxic medications or adjust doses |
| Consider l-carnitine and ursodiol | Not an indication to discontinue pegasparaginase or reduce the dose | |
| Transaminitis | Consider delaying therapy for grades 3 and 4 until resolved to grade 2 | Avoid hepatotoxic medications or adjust doses |
| Consider l-carnitine | Not an indication to discontinue pegasparaginase or reduce dose | |
| Pancreatitis | Early diagnosis and treatment | |
| Supportive medical care | Avoid administering pegasparaginase or any other formulation of asparaginase after clinical asparaginase-associated pancreatitis | |
| Further avoid asparaginase therapy of any form | ||
| No intervention for chemical pancreatitis in the absence of clinical or imaging features | ||
| Hypertriglyceridemia | Consider gemfibrozil | Not an indication to discontinue pegasparaginase |
| Thrombosis | Anticoagulation “not clear” | ATIII replacement for low activity level is not yet standard |
| Maintain adequate platelet counts while patient is receiving anticoagulation | Prophylactic anticoagulation is controversial | |
| Not an indication to discontinue pegasparaginase | ||
| Avoid replacement with cryoprecipitate to correct laboratory abnormalities in the absence of clinical bleed | ||
| Hypofibrinogenemia | Cryoprecipitate replacement only during active bleeding or before procedures | Not an indication to discontinue pegasparaginase |
| Hyperglycemia | Insulin and other anti-glycemic medications | Not an indication to discontinue pegasparaginase |
| Toxicity . | Management . | Prevention . |
|---|---|---|
| Hypersensitivity | Administer corticosteroid and antihistamine | Pre-medicate with hydrocortisone and antihistamine |
| Replace future doses of l-asparaginase with Erwinia asparaginase | Infuse slowly over 2 h | |
| Hyperbilirubinemia | Adjust other medications and delay subsequent cycle until grade 1 is achieved | Avoid hepatotoxic medications or adjust doses |
| Consider l-carnitine and ursodiol | Not an indication to discontinue pegasparaginase or reduce the dose | |
| Transaminitis | Consider delaying therapy for grades 3 and 4 until resolved to grade 2 | Avoid hepatotoxic medications or adjust doses |
| Consider l-carnitine | Not an indication to discontinue pegasparaginase or reduce dose | |
| Pancreatitis | Early diagnosis and treatment | |
| Supportive medical care | Avoid administering pegasparaginase or any other formulation of asparaginase after clinical asparaginase-associated pancreatitis | |
| Further avoid asparaginase therapy of any form | ||
| No intervention for chemical pancreatitis in the absence of clinical or imaging features | ||
| Hypertriglyceridemia | Consider gemfibrozil | Not an indication to discontinue pegasparaginase |
| Thrombosis | Anticoagulation “not clear” | ATIII replacement for low activity level is not yet standard |
| Maintain adequate platelet counts while patient is receiving anticoagulation | Prophylactic anticoagulation is controversial | |
| Not an indication to discontinue pegasparaginase | ||
| Avoid replacement with cryoprecipitate to correct laboratory abnormalities in the absence of clinical bleed | ||
| Hypofibrinogenemia | Cryoprecipitate replacement only during active bleeding or before procedures | Not an indication to discontinue pegasparaginase |
| Hyperglycemia | Insulin and other anti-glycemic medications | Not an indication to discontinue pegasparaginase |
Acknowledgment
The authors thank Mary Clark for assistance in editing the manuscript.
Authorship
Contribution: I.A. and D.D. designed the research, analyzed the data, wrote the manuscript, and approved the final version.
Conflict-of-interest disclosure: D.D. serves on the speakers’ bureau for Servier Pharmaceuticals. I.A. served on the speakers’ bureau with Jazz Pharmaceuticals.
Correspondence: Dan Douer, Division of Hematology, University of Southern California, 1441 Eastlake Ave, Los Angeles, CA 90033; e-mail: douer_d@med.usc.edu.