TO THE EDITOR:
Kaposi sarcoma (KS) herpesvirus (KSHV), also known as human herpesvirus-8, is the causative agent for KS and a plasmablastic form of multicentric Castleman disease (MCD; KSHV-MCD).1 KSHV-MCD is a relapsing and remitting B-cell lymphoproliferative disorder that occurs primarily in people living with HIV.2,3 Patients present with anemia, thrombocytopenia, and inflammatory symptoms associated with elevated cytokines including interleukin-6 (IL-6) and IL-10, elevated levels of C-reactive protein (CRP), and elevated KSHV viral loads (KSHV-VLs). KSHV encodes for viral IL-6 (vIL-6), an analog of human IL-6 (hIL-6), which contributes to KSHV-MCD pathogenesis, in part by driving production of hIL-6.4-6 Treatment options include rituximab (a humanized monoclonal antibody against CD20), rituximab with liposomal doxorubicin or etoposide, or virus-activated cytotoxic therapy with high-dose zidovudine (AZT) and valganciclovir (VGC).7-13 However, some patients do not respond to rituximab, and, when used alone, it is associated with worsening or development of KS in 35% to 67%.8,9
The IL-6 coreceptor (glycoprotein 80 [gp80]) is required for hIL-6 signaling and is a rational B-cell–sparing target for KSHV-MCD. Tocilizumab is a humanized anti-gp80 antibody that inhibits membrane-bound and soluble gp80 signal transduction. It is approved for rheumatoid arthritis and has demonstrated clinical benefit in KSHV− MCD.14,15 Although KSHV-encoded vIL-6 can signal through the gp130 receptor without requiring gp80,16,17 mouse models demonstrated that IL-6 is required for development of clinical features in the setting of vIL-6 excess.6 We hypothesized that blocking hIL-6 signaling would be sufficient to control KSHV-MCD flares. We considered siltuximab, an anti–hIL-6 monoclonal antibody, but found that it does not bind to vIL-6 (supplemental Figure 1, available on the Blood Web site). Additionally, promising results were seen in a tocilizumab trial that included 2 HIV− patients with KSHV-MCD.15 With this background, we initiated an open-label, single-center pilot study to evaluate the clinical benefit of tocilizumab in patients with symptomatic KSHV-MCD and included the option of adding AZT/VGC if tocilizumab alone did not lead to adequate responses.
Adults with pathologically confirmed KSHV-MCD and at least 1 clinical symptom (such as persistent fever, fatigue, gastrointestinal symptoms) and laboratory abnormality (anemia, hypoalbuminemia, thrombocytopenia, or elevated CRP) attributable to KSHV-MCD were eligible. Patients with HIV infection were required to use effective combination antiretroviral therapy. Tocilizumab (8 mg/kg; Roche) was administered IV on day 1 of 14-day cycles for a maximum of 6 cycles (12 weeks). Oral AZT (600 mg every 6 hours) and VGC (900 mg every 12 hours) were administered during days 1 to 5 of a 14-day cycle in combination with tocilizumab where patients had an inadequate response or relapse on tocilizumab monotherapy. Responses were evaluated using the KSHV-MCD Clinical Benefit Response Criteria (CBR), which uses 8 indicator abnormalities (4 symptoms and 4 laboratory findings) closely associated with KSHV-MCD activity and adapted from previous National Cancer Institute (NCI)-MCD response criteria published by our group (supplemental Table 1).7,10 The criteria for the addition of AZT/VGC was elevated CRP (>3 mg/L) and progressive disease by CBR from entry, or failure to achieve partial response (PR) by cycle 4 of tocilizumab monotherapy. The addition of AZT/VGC did not alter the number of tocilizumab doses. Patients with concurrent KS were assessed using the previously described modified AIDS Clinical Trial Group criteria.18 The primary objective was to estimate the clinical benefit of tocilizumab in patients with symptomatic KSHV-MCD using CBR. We also evaluated the benefit and tolerability of the addition of AZT/VGC in cases in which tocilizumab monotherapy was inadequate in achieving or sustaining a response. Exploratory analyses of differences in laboratory findings and serum cytokines between baseline and 48 hours, 2 cycles, and end of treatment were evaluated using the Wilcoxon signed rank test. Progression-free survival (PFS) for all patients was determined using Kaplan-Meier methods from baseline to a subsequent KSHV-MCD flare necessitating further treatment. The protocol (NCT01441063) and accompanying KSHV-MCD natural history protocol (NCT00092222) were approved by the NCI Institutional Review Board. All patients provided written informed consent in accordance with the Declaration of Helsinki.
Between 2011 and 2018, 8 cisgender patients (7 male and 1 female) with symptomatic KSHV-MCD and HIV were enrolled (Table 1). All were on antiretroviral therapy. Four patients (50%) had no prior KSHV-MCD therapy. Two patients had active concurrent KS (25%); 1 patient received prior KS therapy. Five of 8 patients (63%; 95% confidence interval, 25% to 92%) experienced a response as assessed by the modified KSHV-MCD CBR with tocilizumab alone. Best responses on tocilizumab alone were PRs in 4 patients and complete response (CR) in 1 patient (Table 1). Among the 5 tocilizumab-monotherapy responders, beneficial CBR responses were seen within the first 2 cycles (4 weeks) of treatment. Patient 4 (case report previously published [Manion et al19 ]), who had a concurrent diagnosis and treatment of mycobacteria immune reconstitution inflammatory syndrome, had rapid improvement in symptoms and normalization of KSHV-VL, experiencing a CR after cycle 5. She had no further recurrence of KSHV-MCD.
Baseline characteristics, study treatments, and response
| Baseline characteristics . | Study treatment and response . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID . | Sex/Race . | Age at study entry, y . | Concurrent KS/(Stage) . | Time from HIV Dx, mo . | Time from MCD Dx, mo . | Prior therapy for KSHV-MCD or KS . | No. of Tx cycles . | Best response on TCZ alone . | Addition of AZT/ VGC* . | Best response on TCZ +AZT/ VGC . | Response at EOT . | Subsequent therapy . | Time to next therapy, mo . |
| 1 | M/Black | 50 | N | 189 | 107 | Rituximab monotherapy | 6 | PR | Y from C4 | PR | PR | Rituximab | 4 |
| Rituximab/LD | |||||||||||||
| 2 | M/Asian | 47 | N | 51 | 4 | Paclitaxel | 5 | PR | Y from C4 | PR | PD | Rituximab/ LD | 2 |
| 3 | M/Black | 28 | N | 25 | 2 | None | 6 | PD | Y from C2 | CR | CR | Rituximab/ LD | 8 |
| 4 | F/Black | 30 | N | 2 | 1 | Prednisone | 6 | CR | N | — | CR | None | — |
| 5 | M/White | 63 | Y/(T1I0S1) | 108 | 52 | Rituximab, vinorelbine, rituximab/LD, carfilzomib, sirolimus, LD | 6 | PR | N | — | PR | Pom/LD | 4 |
| 6 | M/White | 42 | Y/(T1I1S1) | 5 | 1 | None | 2 | PR | N | — | PD | Pom/LD | 1 |
| 7 | M/White | 64 | N | 206 | 18 | Rituximab | 6 | SD | N | — | PD | Rituximab | 3 |
| 8 | M/Black | 54 | N | 279 | 12 | None | 2 | SD | N | — | PD | Rituximab | 1 |
| 49† | — | 79† | 8† | — | 6† | — | — | — | — | — | 3† | ||
| Baseline characteristics . | Study treatment and response . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID . | Sex/Race . | Age at study entry, y . | Concurrent KS/(Stage) . | Time from HIV Dx, mo . | Time from MCD Dx, mo . | Prior therapy for KSHV-MCD or KS . | No. of Tx cycles . | Best response on TCZ alone . | Addition of AZT/ VGC* . | Best response on TCZ +AZT/ VGC . | Response at EOT . | Subsequent therapy . | Time to next therapy, mo . |
| 1 | M/Black | 50 | N | 189 | 107 | Rituximab monotherapy | 6 | PR | Y from C4 | PR | PR | Rituximab | 4 |
| Rituximab/LD | |||||||||||||
| 2 | M/Asian | 47 | N | 51 | 4 | Paclitaxel | 5 | PR | Y from C4 | PR | PD | Rituximab/ LD | 2 |
| 3 | M/Black | 28 | N | 25 | 2 | None | 6 | PD | Y from C2 | CR | CR | Rituximab/ LD | 8 |
| 4 | F/Black | 30 | N | 2 | 1 | Prednisone | 6 | CR | N | — | CR | None | — |
| 5 | M/White | 63 | Y/(T1I0S1) | 108 | 52 | Rituximab, vinorelbine, rituximab/LD, carfilzomib, sirolimus, LD | 6 | PR | N | — | PR | Pom/LD | 4 |
| 6 | M/White | 42 | Y/(T1I1S1) | 5 | 1 | None | 2 | PR | N | — | PD | Pom/LD | 1 |
| 7 | M/White | 64 | N | 206 | 18 | Rituximab | 6 | SD | N | — | PD | Rituximab | 3 |
| 8 | M/Black | 54 | N | 279 | 12 | None | 2 | SD | N | — | PD | Rituximab | 1 |
| 49† | — | 79† | 8† | — | 6† | — | — | — | — | — | 3† | ||
—, not applicable; C2, cycle 2; C4, cycle 4; Dx, diagnosis; EOT, end of treatment; F, female; ID, identification number; LD, liposomal doxorubicin; M, male; mo, months; N, No; PD, progressive disease; Pom, pomalidomide; SD, stable disease; TCZ, tocilizumab; Tx, treatment; Y, yes; y, years.
C4 and C2 denote cycle when AZT/VGC started.
Median.
Three patients had AZT/VGC added to tocilizumab, resulting in either PR (2 patients) or CR (1 patient) within 4 weeks of combination treatment (Table 1). Clinical benefit was noted in all 3 patients during the first cycle with AZT/VGC, with decreases in KSHV-VL, IL-6, and CRP levels (supplemental Figure 2). Responses were sustained among 2 of 3 patients at the end-of-treatment assessment, whereas 1 patient (patient 2) had progressive KSHV-MCD flare symptoms prior to cycle 6. Three patients did not receive AZT/VGC following incomplete tocilizumab responses due to worsening pulmonary KS (patient 6), thrombocytopenia prohibiting the initiation of AZT/VGC (patient 7), or rapidly progressive KSHV-MCD symptoms (patient 8).
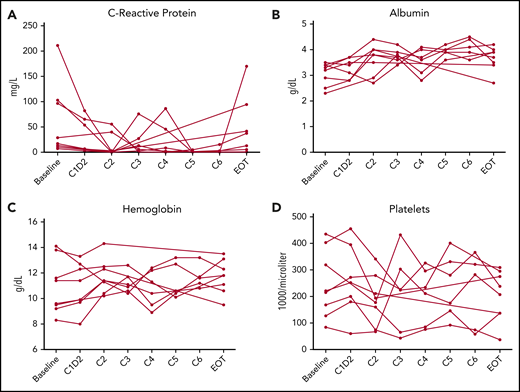
There were decreases in CRP at 48 hours (median, −10.3 mg/L [interquartile range, −49.2 to −5.4 mg/L]; P = .02) in all 7 assessed patients; however, these changes did not consistently persist (Figure 1; supplemental Table 2). Although there was an expected increase in soluble IL-6 and h-IL-6 due to blocking of receptor binding, other cytokines and laboratory parameters associated with KSHV-MCD activity did not change significantly as compared with baseline. vIL-6 messenger RNA levels measured in peripheral blood mononuclear cells, assessed at baseline, cycles 2 and 6, highlighted varying levels of expression among all patients. The messenger RNA vIL-6 transcripts appeared to mirror the KSHV-VL levels in 6 of the 8 patients (supplemental Figure 3).
KSHV-MCD clinical benefit response criteria laboratory variables. (A-D) Four indicator laboratory variables (A, C-reactive protein; B, albumin; C, hemoglobin; D, platelets) in the KSHV-MCD clinical benefit response criteria for 6 cycles (C1-C6) of treatment of all patients over all time points during the study. C1D2, cycle 1 day 2; EOT, end-of-treatment, 2 weeks after the last dose of tocilizumab on study.
KSHV-MCD clinical benefit response criteria laboratory variables. (A-D) Four indicator laboratory variables (A, C-reactive protein; B, albumin; C, hemoglobin; D, platelets) in the KSHV-MCD clinical benefit response criteria for 6 cycles (C1-C6) of treatment of all patients over all time points during the study. C1D2, cycle 1 day 2; EOT, end-of-treatment, 2 weeks after the last dose of tocilizumab on study.
Two patients with concurrent KS had KS progression, and 1 patient developed cutaneous KS with tocilizumab and AZT/VGC treatment on study. The median PFS was 3.2 months for all study participants (95% confidence interval, 1-8.3 months; supplemental Figure 4). Seven patients received rituximab-based regimens or pomalidomide and liposomal doxorubicin for subsequent flares (Table 1). Treatment in this study was generally well tolerated; the most common grade 2 and 3 adverse events possibly, probably, or definitely attributed to treatment were thrombocytopenia and neutropenia for patients treated with tocilizumab monotherapy or in combination with AZT/VGC (supplemental Table 3).
In conclusion, tocilizumab is safe and has activity among patients with a diagnosis of KSHV-MCD and HIV. However, unlike treatment with rituximab, which can yield sustained responses,12 the PFS in this study was short, and tocilizumab did not alter KSHV-VL, IL-10, or IL-1β levels. Incomplete responses may occur because tocilizumab does not block signaling by vIL-6, which binds directly to the gp130 subunit without requiring gp80. Nonetheless, tocilizumab may be useful in the management of KSHV-MCD as part of a multiagent regimen.
Presented in part at the 60th annual meeting of the American Society of Hematology, San Diego, CA, 1-4 December 2018.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank the individuals who volunteered, and the medical, nursing, pharmacy, data management, and support staff of the National Cancer Institute as well as the National Institutes of Health Clinical Center. The authors also thank Kirsta Waldon for work in coordinating the trial, and Randy Stevens and Adam Rupert in Leidos, Frederick, for performing the cytokine assays.
This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health and, in part, by the Intramural Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services (contract \HHSN261200800001E). This project has also been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under (contract No. 75N91019D00024).
Authorship
Contribution: T.S.U., P.G., and R.Y. designed the study; A.W., V.M., W.D.F., C.J.P., D.W., V.W., J.Z., A.S., J.G., and R.R. collected and analyzed the data; A.W., P.G., T.S.U., R.R., K.L., and R.Y. cared for the patients on the study; S.M.S. analyzed data and provided statistical support; and R.R., K.L., T.S.U., and R.Y. wrote the manuscript.
Conflict-of-interest disclosure: R.R., T.S.U., K.L., and R.Y. report receiving research support from Celgene through a Cooperative Research and Development Agreement (CRADA) at the National Cancer Institute (NCI) and drugs for a clinical trial from Merck and EMD Serono through a CRADA with the NCI. T.S.U. reports receiving other commercial research support from Roche through a clinical trial agreement (CTA) with Fred Hutchinson Cancer Research Center. T.S.U., R.Y., and D.W. are co-inventors on US patent 10 001 483 entitled, “Methods for the treatment of Kaposi’s sarcoma or KSHV-induced lymphoma using immunomodulatory compounds, and uses of biomarkers.” R.Y. is also a co-inventor on patents on a peptide vaccine for HIV and on the treatment of KS with IL-12, and has an immediate family member who is a co-inventor on patents related to internalization of target receptors, on KSHV vIL-6, and on the use of calreticulin and calreticulin fragments to inhibit angiogenesis. All rights, title, and interest to these patents have been or should by law be assigned to the US Department of Health and Human Services; the government conveys a portion of the royalties it receives to its employee inventors under the Federal Technology Transfer Act of 1986 (PL 99-502). The remaining authors declare no competing financial interests.
Correspondence: Ramya Ramaswami, HIV/AIDS Malignancy Branch, Center for Cancer Research, 10 Center Dr, 6N106, Bethesda, MD 20892; e-mail: ramya.ramaswami@nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal