Key Points
PICCs have an increased rate of VTE over TLs in children.
Children with a history of thrombosis, leukemia, or a multilumen CVC have an increased risk of VTE.
Abstract
Venous thromboembolism (VTE) incidence in children has sharply increased with the majority of cases secondary to central venous catheters (CVCs). Among CVCs, the number of peripherally inserted central catheters (PICCs) placed has risen significantly. In this multicenter, prospective, observational cohort study, we enrolled patients aged 6 months to 18 years with newly placed PICCs or tunneled lines (TLs). We evaluated the incidence of VTE, central line–associated bloodstream infections (CLABSIs), and catheter malfunctions in PICCs and TLs, and risk factors of CVC-related VTE. A total of 1967 CVCs were included in the analysis. The incidence of CVC-related VTE was 5.9% ± 0.63%. The majority of the cases, 80%, were in subjects with PICCs, which had a significantly higher risk of catheter-related VTE than subjects with TLs (hazard ratio [HR] = 8.5; 95% confidence interval [CI], 3.1-23; P < .001). PICCs were significantly more likely to have a CLABSI (HR = 1.6; 95% CI, 1.2-2.2; P = .002) and CVC malfunction (HR = 2.0; 95% CI, 1.6-2.4; P < .001). Increased risk of CVC-related VTE was found in patients with a prior history of VTE (HR = 23; 95% CI, 4-127; P < .001), multilumen CVC (HR = 3.9; 95% CI, 1.8-8.9; P = .003), and leukemia (HR = 3.5; 95% CI, 1.3-9.0; P = .031). Children with PICCs had a significantly higher incidence of catheter-related VTE, CLABSI, and CVC malfunction over TLs. The results suggest that pause be taken prior to placing CVCs, especially PICCs, due to the serious complications they have been shown to cause.
Medscape Continuing Medical Education online
In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 231.
Disclosures
Associate Editor Thomas L. Ortel served as advisor or consultant for Instrumentation Laboratory and received grants for clinical research from Instrumentation Laboratory and Siemens. CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC and the authors declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Compare venous thromboembolism (VTE) incidence between children with newly placed peripherally inserted central catheters (PICCs) and tunneled lines (TLs), according to a multicenter, prospective observational cohort study in patients ages 6 months to 18 years
Describe risk factors for central venous catheter (CVC)–related VTE, central line–associated bloodstream infections and catheter malfunctions in children with newly placed CVCs, according to this multicenter, prospective observational cohort study
Identify clinical implications of VTE incidence in children with newly placed PICCs or TLs, according to this multicenter, prospective observational cohort study
Release date: January 16, 2020; Expiration date: January 16, 2021
Introduction
Central venous catheters (CVCs) are necessary and important devices often required in the care of medically complex and acutely ill children.1 They provide access to the venous system for infusions of medications, blood products, and fluids as well as painless withdrawal of blood for laboratory testing. Unfortunately, these devices can lead to serious complications such as venous thromboembolism (VTE) and central line–associated bloodstream infections (CLABSIs).2-4 Historically, VTE, consisting of deep venous thrombosis and pulmonary embolism, has been rare in children, but recent data demonstrate that the incidence of VTE has increased by 70% to 200% in the last 2 decades with ∼80% of cases in children caused by CVCs.5-7
The most commonly placed CVCs in children, aimed at providing intermediate to long-term vascular access, are peripherally inserted central catheters (PICCs) and tunneled lines (TLs). The main variance between these CVCs is their insertion site, which leads to differences in several relevant factors when considering the risk of thrombosis. PICCs are placed into vessels of smaller caliber and their intravascular course traverses from the mid-arm or antecubital fossa to the superior vena cava (SVC) or SVC/right-atrial (RA) junction. In contrast, TLs are inserted into large central veins and their intravascular course has a comparatively shorter distance. Physiologically, PICCs occupy a greater portion of the vessel lumen than TLs and thus there is a theoretical increased VTE risk.8 Due to the ability to place and remove PICCs at bedside with little or no sedation, compared with TLs, PICC insertion rates have increased significantly in recent years.9
The reported incidence of CVC-related VTE has been highly variable, ranging from 2% to 81%.4,10 Previous studies focused on limited patient populations, such as infants, those in the intensive care setting, or patients with a malignancy.11-13 Studies have also been predominantly retrospective, single institution, or have evaluated a single CVC type.4,12,14 Prospective studies included predominantly asymptomatic VTE cases, which may not be clinically significant.11,15 With this in mind, we set out to definitively evaluate the incidence rate of imaging confirmed, symptomatic, catheter-related VTE in subjects with newly placed PICCs compared with TLs in this multicenter Clot Incidence Rates in Central Lines (CIRCLE) study. Due to the longer intravascular course within smaller vessels, we hypothesized that PICCs would have a higher VTE rate than TLs.
Methods
Study design
The CIRCLE study was a multicenter, prospective, observational cohort study conducted at 4 tertiary care centers throughout the United States from October 2013 to June 2018. The study included subjects from Children’s Hospital Los Angeles, Children’s Hospital of Philadelphia, Nationwide Children’s Hospital, and Texas Children’s Hospital. The study was approved by the institutional review board and the ethics committee at each institution and a waiver of consent was granted. Data quality was internally audited for accuracy and completeness at each institution.
Study objectives
Our primary objective was to compare the incidence rate and risk factors of CVC-related VTE in PICCs and TLs in children. Secondary objectives were to compare the incidence of CLABSIs and catheter malfunctions between PICCs and TLs. For subjects diagnosed with a VTE, rates of CLABSI and malfunction were compared prior to the diagnosis of VTE.
Study population
Eligible subjects were children aged 6 months to less than 18 years with a newly placed PICC or TL. TLs included fully implanted Port-a-cath as well as external cuffed devices such as a Broviac, Hickman, Medcomp, and Powerline. Subjects who had >1 CVC placed during the 5-year study period were eligible to be reenrolled if they were not diagnosed with a VTE during their first enrollment. Subjects younger than 6 months of age were excluded from the study due to differences in developmental coagulation that may have effects on study outcomes, as well as typically being too small to have TLs placed; thus, the main outcome of the study could not be compared in this group. Subjects were also excluded if they were currently being treated for a VTE.
Study procedures
Subjects were randomly selected and enrolled in the study through daily or weekly reports of patients with newly placed CVCs at each institution. After an interim analysis identified enrollment of subjects heavily weighted toward PICCs (due to the high volume of PICC placement), TLs and PICCs were enrolled in a 1:1 fashion after March 2017. During the random subject selection, differences in demographics, medical history, or reason for CVC placement between subject populations were not taken into account. The subjects’ primary medical team determined the type of CVC placed, and placement was performed according to the standard of care at each institution. From the day of CVC placement, subjects were monitored prospectively via electronic medical record review for the development of a VTE, CLABSI, or CVC malfunction. All diagnoses of VTE required radiological confirmation, and no screening imaging was performed for the study. Symptomatic VTE was defined as subjects having pain, swelling, numbness, erythema, unexplained fever, transaminitis or thrombocytopenia, and discoloration or change in temperature in their limb with a CVC in place. Subjects were defined as having a CLABSI if an organism was cultured from the blood from a CVC that was in place for >2 days without having an infection at another site. A CVC malfunction was defined as a blockage in the CVC requiring treatment with an instillation of tissue plasminogen activator.
Data characteristics
Subject data were collected using standardized case report forms through Research Electronic Data Capture (REDCap), a free, secure, web-based application. Data collected included subject demographics, medical history, CVC characteristics (catheter size, number of lumens, catheter material, and brand) and CVC placement procedure details (vein accessed and attempts). For any subject diagnosed with a catheter-related VTE, symptoms, initial diagnostic imaging, treatment, follow-up imaging, and adverse event data were collected. All decisions regarding management of catheter-related complications were made by the medical team.
Statistical methods
The rate of catheter-related VTE, CLABSI, CVC malfunction, and line removal was estimated and compared between PICCs and TLs. A VTE was considered to be associated with the CVC if the VTE occurred before or within 30 days of line removal. For the analysis of CLABSI or malfunction, events that occurred after the date of line removal were not included with this analysis.
With a goal sample size of 2000 CVC placements, including 6 months of additional follow-up, 10% censoring per year, and an overall VTE rate of ∼5%, this study was calculated to have a power of at least 0.80 to detect a relative failure rate of 3.6 if PICCs were associated with higher VTE rates, or a relative failure rate of 2.7 if TLs were associated with higher VTE rates with 2-sided type I error of 0.05.
The association between subject and CVC characteristics and the risk of having a catheter-associated VTE were examined using parametric survival models assuming a Weibull survival distribution.16 These analyses used each CVC insertion and its follow-up data rather than subjects as the primary analytic unit, so each subject could contribute data on 1 or more CVCs. This intrapatient correlation was handled with shared frailty in the survival analyses.16,17 When assessing the association between CLABSI or CVC malfunction and the risk of having a catheter-related VTE, CLABSI or CVC malfunction was analyzed as a time-dependent covariate in the survival analyses. When there were multiple CLABSIs or malfunctions associated with 1 CVC, only the first occurrence events were considered in the analyses.
The association between occurrence of catheter-related VTE and subject/CVC characteristic was first assessed in univariate analyses and then assessed by a minimal multivariable model. The variables that were associated with catheter-related VTE diagnosis at P ≤ .35 in the univariate analyses were included in a base model, and the backward selection procedure was used to eliminate any variable that was not significant at P ≤ .10.18 All P values are 2 sided. Statistical analyses were performed using STATA software (version 11.0; StataCorp LP, College Station, TX).19
Results
Characteristics of subjects
From October 2013 to June 2018, a total of 2006 CVC placements were enrolled. Thirty-nine subjects were excluded from the analysis leaving 1967 CVCs placed in 1742 unique subjects (Figure 1).
Reasons for exclusion of subjects from the final analysis. The majority of exclusions were due to subjects being diagnosed with a CVC-related VTE and then reenrolled on the study a second time with a new CVC placement.
Reasons for exclusion of subjects from the final analysis. The majority of exclusions were due to subjects being diagnosed with a CVC-related VTE and then reenrolled on the study a second time with a new CVC placement.
One hundred eighty-three subjects had 2 or more CVCs placed during the study period. The median subject age at CVC insertion was 6.4 years (range, 0.6-17.9 years) and included 1047 male patients (53%) and 920 female patients (47%). Cancer was the predominate diagnosis of subjects enrolled in the study (802; 41%). Complete demographic information for the study population is provided in Table 1.
Comparison of demographic information on subjects with either a PICC or TL at the time of catheter placement
| Variables . | PICC group, n = 1257 . | TL group, n = 710 . |
|---|---|---|
| Median (range) or n (%) . | Median (range) or n (%) . | |
| Age at CVC insertion, y | 7 (0.5-17.9) | 5.3 (0.5-17.9) |
| Age group, y | ||
| 0.5-1 | 101 (8) | 36 (5) |
| >1-5 | 398 (32) | 312 (44) |
| >5-10 | 300 (24) | 148 (21) |
| >10-18 | 458 (36) | 214 (30) |
| Sex | ||
| Female | 603 (48) | 317 (45) |
| Male | 654 (52) | 393 (55) |
| Ethnicity | ||
| Hispanic/Latino | 429 (34) | 231 (33) |
| Non-Hispanic | 508 (40) | 302 (42) |
| Not listed | 320 (26) | 177 (25) |
| Race | ||
| White | 585 (47) | 375 (53) |
| Asian | 71 (6) | 54 (8) |
| Black or African American | 157 (12) | 70 (10) |
| Native Hawaiian/Pacific Islander, American Indian, or Native Alaskan | 6 (<1) | 16 (2) |
| Unknown | 438 (35) | 195 (27) |
| Medical history* | ||
| Current infection | 423 (34) | 95 (13) |
| Previous CVC | 375 (30) | 291 (41) |
| Recent surgery | 287 (23) | 145 (20) |
| Cancer | 268 (21) | 534 (75) |
| Parental nutrition dependence | 207 (16) | 101 (14) |
| Congenital heart disease | 118 (9) | 11 (2) |
| History of thrombosis | 93 (7) | 49 (7) |
| Cystic fibrosis | 84 (7) | 8 (<1) |
| Metabolic/mitochondrial disorder | 69 (5) | 24 (3) |
| Inflammatory bowel disease | 34 (3) | 5 (<1) |
| Nephrotic syndrome | 11 (<1) | 4 (<1) |
| Lupus/juvenile rheumatoid arthritis | 8 (<1) | 2 (<1) |
| None/other | 230 (18) | 41 (6) |
| Type of TL | ||
| Port-a-cath | † | 484 (68) |
| Broviac | † | 125 (18) |
| Hickman | † | 62 (9) |
| Medcomp | † | 18 (3) |
| Powerline | † | 3 (<1) |
| Unknown/other | † | 18 (3) |
| Variables . | PICC group, n = 1257 . | TL group, n = 710 . |
|---|---|---|
| Median (range) or n (%) . | Median (range) or n (%) . | |
| Age at CVC insertion, y | 7 (0.5-17.9) | 5.3 (0.5-17.9) |
| Age group, y | ||
| 0.5-1 | 101 (8) | 36 (5) |
| >1-5 | 398 (32) | 312 (44) |
| >5-10 | 300 (24) | 148 (21) |
| >10-18 | 458 (36) | 214 (30) |
| Sex | ||
| Female | 603 (48) | 317 (45) |
| Male | 654 (52) | 393 (55) |
| Ethnicity | ||
| Hispanic/Latino | 429 (34) | 231 (33) |
| Non-Hispanic | 508 (40) | 302 (42) |
| Not listed | 320 (26) | 177 (25) |
| Race | ||
| White | 585 (47) | 375 (53) |
| Asian | 71 (6) | 54 (8) |
| Black or African American | 157 (12) | 70 (10) |
| Native Hawaiian/Pacific Islander, American Indian, or Native Alaskan | 6 (<1) | 16 (2) |
| Unknown | 438 (35) | 195 (27) |
| Medical history* | ||
| Current infection | 423 (34) | 95 (13) |
| Previous CVC | 375 (30) | 291 (41) |
| Recent surgery | 287 (23) | 145 (20) |
| Cancer | 268 (21) | 534 (75) |
| Parental nutrition dependence | 207 (16) | 101 (14) |
| Congenital heart disease | 118 (9) | 11 (2) |
| History of thrombosis | 93 (7) | 49 (7) |
| Cystic fibrosis | 84 (7) | 8 (<1) |
| Metabolic/mitochondrial disorder | 69 (5) | 24 (3) |
| Inflammatory bowel disease | 34 (3) | 5 (<1) |
| Nephrotic syndrome | 11 (<1) | 4 (<1) |
| Lupus/juvenile rheumatoid arthritis | 8 (<1) | 2 (<1) |
| None/other | 230 (18) | 41 (6) |
| Type of TL | ||
| Port-a-cath | † | 484 (68) |
| Broviac | † | 125 (18) |
| Hickman | † | 62 (9) |
| Medcomp | † | 18 (3) |
| Powerline | † | 3 (<1) |
| Unknown/other | † | 18 (3) |
Some subjects had >1 medical history.
Sample size represents CVCs not subjects because some subjects were reenrolled when a new CVC was placed.
CVC characteristics
Of the 1967 CVCs included, 1257 (64%) were PICCs and 710 (36%) were TLs. The most common type of TL placed was a Port-a-cath (n = 484; 70%). The majority of PICCs were inserted into the basilic vein (n = 694; 56%), with most of the remainder inserted into the brachial vein (n = 326; 26%). TLs were most commonly placed in the internal jugular vein (n = 385; 55%) and the subclavian vein (n = 288; 41%). The tip of the CVC was located in the SVC/RA junction in 1097 subjects (56%), SVC in 566 subjects (29%), and RA in 167 subjects (8%). The majority of the CVCs, 1228 (62%), were single lumen.
VTE events
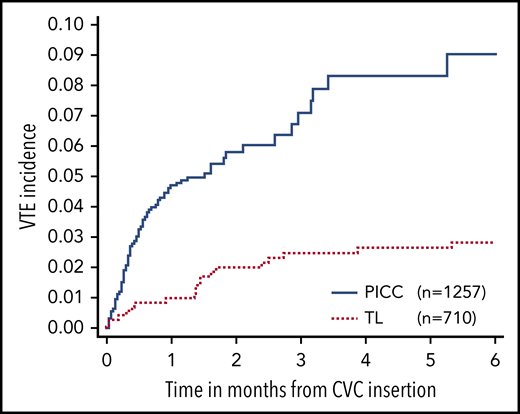
Ninety-four of the 1967 CVCs placed had a diagnosis of a VTE within 6 months of CVC insertion. Doppler ultrasonography was used to diagnose 84 cases (94%), and 41 cases (44%) had completely occluded vessels. The overall incidence rate of catheter-related VTE was 5.9% ± 0.63%. Seventy-five (80%) of the catheter-related VTE events were in PICCs, with an incidence rate of 9.0% ± 1.4% (Figure 2). TLs had a catheter-related VTE incidence rate of 2.9% ± 0.64% and subjects with PICCs had a significantly higher risk of catheter-related VTE than subjects with TLs (hazard ratio [HR] = 8.5; 95% confidence interval [CI], 3.1, 23; P < .001).
Comparison of CVC-related VTE events in PICCs vs TLs within 6 months after CVC insertion.
Comparison of CVC-related VTE events in PICCs vs TLs within 6 months after CVC insertion.
The median time from CVC insertion (all CVC types) to VTE diagnosis was 15.5 days (range, 1-162 days). In PICCs, the median time to VTE diagnosis was 14 days (range, 1-160 days) compared with 42 days (range, 1-162 days) in TLs.
Central line–related blood stream infection and malfunction events
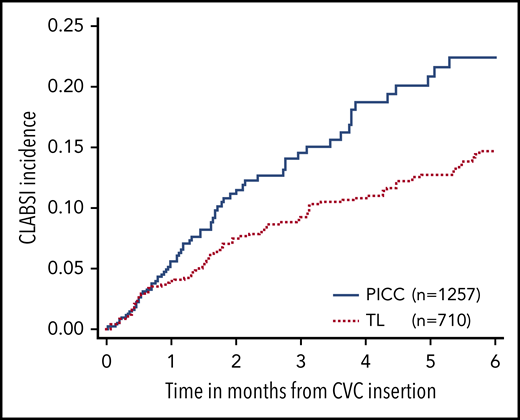
One hundred seventy-three CLABSI events were identified from 158 unique subjects within 6 months of CVC insertion. The incidence rate of CLABSI in all CVC types was 17% ± 1.3%, in which 81 events (incidence, 22% ± 2.8%) were in PICCs and 92 events (incidence, 15% ± 1.4%) were in TLs. PICCs were significantly more likely to have a CLABSI (HR = 1.6; 95% CI, 1.2-2.2; P = .002) than TLs (Figure 3). For lines with a CLABSI, the median time from CVC insertion to a CLABSI was 1.4 months.
Comparison of CLABSI events in PICCs vs TLs within 6 months after CVC insertion.
Comparison of CLABSI events in PICCs vs TLs within 6 months after CVC insertion.
A malfunction occurred in 444 CVCs from 402 unique subjects within 6 months of insertion with an overall incidence rate of 35% ± 1.5%. PICCs were significantly more likely to have a malfunction than TLs (HR = 2.0; 95% CI, 1.6-2.4; P < .001), and the incidence rate was 41% ± 2.7% for PICCs compared with 29% ± 1.8% for TLs.
VTE risk factors
Multivariable analyses revealed a significantly increased risk of CVC-related VTE in subjects with PICCs (HR = 8.5; 95% CI, 3.1-23; P < .001), a prior history of VTE (HR = 23; 95% CI, 4-127; P < .001), a multilumen CVC (HR = 3.9; 95% CI, 1.8-8.9; P = .003), and a diagnosis of leukemia (HR = 3.5; 95% CI, 1.3-9.0; P = .031) (Table 2). CLABSI and CVC malfunction were found to be independently associated with a subject’s risk of developing a VTE (Table 2). Other CVC characteristics, such as CVC tip location, catheter brand, access location, catheter material, French size, CVC length, and number of insertion attempts, were not identified as an increased risk of CVC-related VTE. Patient characteristics such as sex, body mass index, body surface area, race, or ethnicity were also not shown to predict CVC-related VTE risk.
Variables found to have increased risk of developing a CVC-related VTE
| Variables . | n . | VTEs . | Multivariable . | |
|---|---|---|---|---|
| HR (95% CI) . | P . | |||
| CVC type | <.001 | |||
| TLs | 710 | 19 | 1.0 | |
| PICCs | 1257 | 75 | 8.5 (3.1, 23) | |
| History of VTE | <.001 | |||
| No | 1825 | 75 | 1.0 | |
| Yes | 142 | 19 | 23 (4.0, 127) | |
| No. of lumens | .003 | |||
| 1 | 1228 | 41 | 1.0 | |
| 2 or 3 | 625 | 50 | 3.9 (1.8, 8.9) | |
| Unknown | 114 | 3 | ||
| Cancer | .031 | |||
| No cancer | 1165 | 49 | 1.0 | |
| Leukemia/lymphoma | 456 | 33 | 3.5 (1.3, 9.0) | |
| Other cancer | 346 | 12 | 1.3 (0.39, 4.5) | |
| CLABSI | .002 | |||
| No | 1794 | 82 | 1.0 | |
| Yes | 173 | 12 | 5.6 (1.9, 16) | |
| CVC malfunction | <.001 | |||
| No | 1523 | 64 | 1.0 | |
| Yes | 444 | 30 | 5.8 (2.5, 13) | |
| Variables . | n . | VTEs . | Multivariable . | |
|---|---|---|---|---|
| HR (95% CI) . | P . | |||
| CVC type | <.001 | |||
| TLs | 710 | 19 | 1.0 | |
| PICCs | 1257 | 75 | 8.5 (3.1, 23) | |
| History of VTE | <.001 | |||
| No | 1825 | 75 | 1.0 | |
| Yes | 142 | 19 | 23 (4.0, 127) | |
| No. of lumens | .003 | |||
| 1 | 1228 | 41 | 1.0 | |
| 2 or 3 | 625 | 50 | 3.9 (1.8, 8.9) | |
| Unknown | 114 | 3 | ||
| Cancer | .031 | |||
| No cancer | 1165 | 49 | 1.0 | |
| Leukemia/lymphoma | 456 | 33 | 3.5 (1.3, 9.0) | |
| Other cancer | 346 | 12 | 1.3 (0.39, 4.5) | |
| CLABSI | .002 | |||
| No | 1794 | 82 | 1.0 | |
| Yes | 173 | 12 | 5.6 (1.9, 16) | |
| CVC malfunction | <.001 | |||
| No | 1523 | 64 | 1.0 | |
| Yes | 444 | 30 | 5.8 (2.5, 13) | |
CLABSI and CVC malfunction were diagnosed prior to the VTE event.
CI, confidence interval; HR, hazard ratio.
Discussion
Due to their ease of placement and removal at bedside, and decreased insertion complications, PICCs have become the preferred CVC choice when children require central venous access.20,21 Insertion of PICCs in children has increased over the last 2 decades,9 and 1 study recommends placing PICCs in children when >4 days of peripheral IV access is required due to improved patient and family satisfaction rates.21 The previous decade has seen both increased PICC insertion rates and increased incidence of VTE in children. Although the overall increased VTE incidence relates, in part, to more effective and intense treatments for children, the primary driver of this surge of VTE is due to CVCs.22,23 Importantly, children who develop a VTE not only require anticoagulation therapy with its inherent risk and inconvenience, but also experience prolonged hospitalizations, increased cost, and increased likelihood of death, which are also associated with VTE.6,24 Thus, the results of the CIRCLE study should give pause to the community of pediatricians and pediatric specialists who care for children who require IV treatments, especially when central venous access is not obviously necessary, as a VTE rate of 9% from an iatrogenic cause (PICCs) is, in our view, too high to justify the current approach.
In this multi-institutional, prospective cohort study, children with PICCs had a significantly increased risk of developing a catheter-related VTE compared with TLs. PICCs were also more likely to have a CLABSI and malfunction as compared with TLs. Although there are prior studies assessing risk factors for thrombosis in children, the CIRCLE study is by far the largest prospective study of its kind. Furthermore, the CIRCLE study is uniquely addressing an important and clinically relevant clinical issue in pediatrics: the need to fully appreciate the potential negative consequences of PICCs.
In both PICCs and TLs, this study identified multilumen catheters as having an increased risk of thrombosis (HR = 3.9; 95% CI, 1.8-8.9; P = .003), which has been noted in previous studies.12 Multilumen CVCs tend to be larger in caliber, and thus occupy more area within the vessel lumen and cause blockage of normal blood flow within the vein, which can lead to a higher likelihood of thrombosis.25 This study also highlights that children with leukemia and a history of VTE are more likely to develop a catheter-related VTE, which is consistent with previous studies.10,26,27 Children with leukemia have multiple risk factors for VTE development including the prothrombotic nature of cancer itself and the presence of hypercoagulable blast cells, as well as treatment with l-asparaginase chemotherapy and steroids.
We did not find an associated catheter-related VTE risk between various CVC insertion characteristics, such as CVC tip location, insertion attempts, vein accessed, French size, CVC length, access side, catheter brand, or material. Although our study was designed to have sufficient statistical power to compare catheter-related VTE risks between PICCs and TLs, there was limited variation in line-tip placement and number of insertion attempts. For example, the majority, 86%, of CVC tips were placed in the SVC or SVC/RA junction and 96% of the CVCs were inserted within ≤2 attempts; hence, we were unable to statistically compare other tip locations and >2 access attempts.
CLABSIs remain a serious complication with any CVC. Results from this study show that children with PICCs have an increased risk of infection over TLs, and those diagnosed with a CLABSI have an increased risk of developing a catheter-related VTE. However, because we did not perform screening imaging in our study, we cannot rule out the possibility that a VTE was present prior to some of the cases of CLABSI. Previous studies have found that external, cuffed CVCs have an increased rate of infection over implanted CVCs, but this it the first pediatric study showing that PICCs have an increased infection rate over both devices.28 The increased infection rate may be due to the hub of the PICC being in the antecubital fossa, which is difficult to keep dry and clean vs TLs in which a portion of the catheter is threaded into a subcutaneous “tunnel” in the chest.
Besides implementing modifiable risk factors, such as inserting single-lumen CVCs when possible and using sterile technique when accessing lines to prevent CLABSIs, future efforts need to focus on VTE prevention. Unfortunately, the effectiveness of pharmacological prophylaxis to prevent CVC-related VTE in children has not been successful.29,30 Focus should also be aimed at increased evaluation for VTE in patients requiring multiple doses of tissue plasminogen activator due to CVC malfunction. The incidence of CVC-related VTE may have been even higher in this study if subjects with CVC malfunction were regularly evaluated with Doppler ultrasonography.
A potential limitation of the study is the exclusion of temporary, nontunneled CVCs commonly used in critically ill children. These lines are not intended for long-term vascular access and thus were not included in the scope of this study. Another limitation was the exclusion of surveillance imaging to evaluate for thrombosis in asymptomatic subjects, especially those with TLs. Subjects with PICCs are potentially more readily diagnosed with a symptomatic VTE due to the PICCs being placed in smaller vessels and the PICC journey through the arm or leg causing limb pain and swelling vs a TL which lies in the chest. We felt, as stated herein, that the clinical relevance of asymptomatic VTE is unknown; thus, we chose to focus on known, clinically significant VTE cases. The predominant use of Doppler ultrasonography to diagnose upper extremity VTE may have also been a limitation due to potential missed cases, although guidelines from the American Society of Hematology recommend this imaging modality, which is far less invasive and expensive than alternative choices.31 PICCs may have also been placed in more acutely ill patients compared with TLs. We attempted to avoid this confounding variable by comparing a validated pediatric illness severity score or pediatric early warning score in those with PICCs vs TLs,32 but <40% of subjects had a pediatric early warning score performed on the day of CVC placement.
In conclusion, CVCs are an essential part of medical care for many children, but the increasing rates of CVC-related VTE must be addressed. To lower the CVC-related VTE rate, we must focus on modifiable risk factors, such as limiting multilumen CVCs, preventing CLABSIs, or placing a TL over a PICC if possible. Most importantly, pediatricians and pediatric subspecialists need to reconsider how quickly and easily decisions to place CVCs, especially PICCs, are being placed. Inserting a PICC is tempting to avoid repeat venipuncture or surgical intervention with TL placement, but results from the CIRCLE study showing increased risks of VTE, CLABSI, and malfunction with PICCs should give practitioners pause.
For original data, please contact Julie Jaffray at jjaffray@chla.usc.edu.
Presented in abstract form at the 60th annual meeting of the American Society of Hematology, San Diego, CA, 1-4 December 2018.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.J. and G.Y. conceived the study and wrote the study protocol; J.J. was the lead investigator and thus wrote the first draft of the manuscript, which was subsequently edited by all coauthors; and all authors entered patient information, analyzed data, and participated in the writing and editing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Julie Jaffray, Division of Hematology/Oncology/Bone Marrow Transplant, Children’s Hospital Los Angeles, 4650 Sunset Blvd, Mailstop #54, Los Angeles, CA 90027; e-mail: jjaffray@chla.usc.edu.




Comments
E-LETTER FOR JAFFRAY ET AL STUDY, BLOOD 2020
We read with interest the Jaffray et al study in number 3 of the January 16 Blood journal[1].
In their multicenter and observational large cohort study of a prevalently non-hematological (77%) pediatric population on the incidence of peripherally inserted central catheters (PICCs) versus tunneled lines (TLs)-associated venous thromboembolism (VTE) and bloodstream
infection (BSI), PICCs had a significantly higher incidence of VTE and BSI over TLs (9% vs.
2.9%; 22% vs. 15%, respectively). The authors suggested to hold back placing PICCs, as they have shown to cause serious complications. However, we recently published the results of a randomized phase IV clinical trial on 93 adult patients with acute myeloid leukemia, receiving intensive chemotherapy for hematological remission induction, testing the incidence
of PICCs versus centrally inserted (external non-tunneled) central catheters (CICCs)-related VTE and BSI [2]. In our study, PICCs had a significantly lower incidence of VTE and BSI over CICCs (8.7% vs. 25%; 4.3% vs. 23.4%, respectively). PICC type was double lumen 5 Fr in 60% of cases, insertion site was basilic vein in 90% of cases, and tip location was cavo-atrial
junction in 78% of cases. In our trial, a detailed protocol was strictly followed during PICC implantation, which might explain the relatively low incidence of major adverse events. Bilateral ultrasonography scans (US) of all arm and neck veins, and clear identification of the median nerve and brachial artery was required before venipuncture. The upper mid-arm vein
selected (green zone insertion method) was the vessel whose diameter in mm on the US corresponded to the catheter diameter in Fr. Internal jugular vein US evaluation was performed during catheter introduction. Hand washing, an aseptic technique, and maximal barrier protection was strictly performed. Finally, we used an intracavitary electrocardiographic method to assess the tip position and secured the PICC with a sutureless device3
. In conclusion, evidence indicates that PICC use (with a truly mini-invasive
procedure) in a sub-set of high-risk hematological adult patients is safer than CICC [4].
Marco Picardi
Roberta Della Pepa
Claudia Giordano
Novella Pugliese
Fabrizio Pane
References
1. Jaffray J, Witmer C, O'Brien SH, et al. Peripherally inserted central catheters lead to a
high risk of venous thromboembolism in children. Blood. 2020;135(3):220-226.
2. Picardi M, Della Pepa R, Cerchione C, et al. A Frontline Approach With Peripherally
Inserted Versus Centrally Inserted Central Venous Catheters for Remission Induction
Chemotherapy Phase of Acute Myeloid Leukemia: A Randomized Comparison. Clin
Lymphoma Myeloma Leuk. 2019;19(4):e184-e194.
3. Scoppettuolo G PM. Ultrasound guided placement of peripherally inserted central
venous catheters. Philadelphia: Elsevier -Saunders; 2014.
4. Picardi M, Pagliuca S, Chiurazzi F, et al. Early ultrasonographic finding of septic
thrombophlebitis is the main indicator of central venous catheter removal to reduce infectionrelated mortality in neutropenic patients with bloodstream infection. Ann Oncol.
2012;23(8):2122-2128.