In this issue of Blood, Tran Quang et al show treatment with anti-CD3 antibodies produces potent antileukemic effects in mouse xenograft models of disseminated T-cell acute lymphoblastic leukemia (T-ALL).1 Using several high-tumor-burden patient-derived xenograft (PDX) models of T-ALL, the authors demonstrate that 5 daily injections of anti-CD3 antibodies induced temporary regression of leukemia and prolonged mouse survival. Combining the antibody injections with vincristine and dexamethasone further enhanced these effects, resulting in complete eradication of established T-ALL in most animals. These results suggest anti-CD3 antibodies can be explored as a treatment of patients with sCD3+ T-ALL.
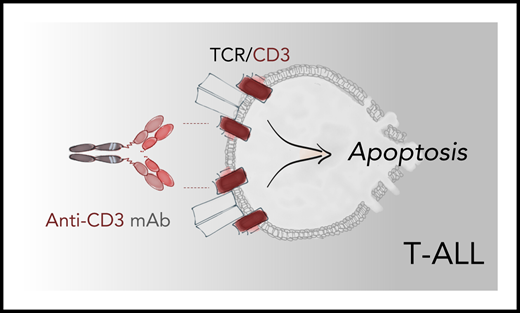
Binding of an anti-CD3 antibody activates TCR signaling in medullary T-ALL leading to apoptosis of leukemic blasts through a program that resembles negative selection of thymocytes. Anti-CD3 antibodies teplizumab and foralumab produced robust antileukemic activity in PDX mouse models of sCD3+ T-ALL, suggesting a new therapeutic avenue for patients with this disease. mAb, monoclonal antibody.
Binding of an anti-CD3 antibody activates TCR signaling in medullary T-ALL leading to apoptosis of leukemic blasts through a program that resembles negative selection of thymocytes. Anti-CD3 antibodies teplizumab and foralumab produced robust antileukemic activity in PDX mouse models of sCD3+ T-ALL, suggesting a new therapeutic avenue for patients with this disease. mAb, monoclonal antibody.
CD3 subunits are expressed on the cell surface primarily in medullary (mature) T-ALL, which comprises about half of all pediatric cases and ∼20% of cases in adults. These leukemic cells resemble mature thymocytes with partially or fully recombined T-cell receptor (TCR) chains that normally undergo positive and negative selection in the thymus. Negative selection occurs when a TCR binds to self-peptide–major histocompatibility complexes with exceedingly high affinity, triggering an apoptosis program that eliminates autoreactive T cells. Previously, the authors demonstrated the ability of a murine anti-CD3 antibody OKT3 (muromonab) to induce a similar TCR signaling in mature T-ALL blasts, directly activating the apoptotic program without utilizing FcR- and complement-dependent cytotoxicity mechanisms.2 In the current study, Tran Quang and colleagues showed teplizumab and foralumab, modified anti-CD3 antibodies with established clinical safety profile, promote robust antitumor activity in T-ALL PDX models by utilizing the mechanism described above (see figure).
Teplizumab is humanized, and foralumab is a fully human CD3ε-specific antibody; both harbor point mutations in the CH2 Fc domain that reduce binding to Fc receptors and complement.3 Thus, these antibodies are less likely to induce cytokine release and the toxicities associated with the use of muromonab in the clinic. Both CD3-specific antibodies can suppress unwanted pathogenic autoimmune and alloimmune T-cell responses by promoting TCR signaling-induced anergy and apoptosis in pathogenic T cells and by stimulating the expansion of regulatory T cells.3,4 Teplizumab and foralumab have shown acceptable safety profiles at lower doses in patients with autoimmune diseases (type 1 diabetes and Crohn disease).5,6 Following corticosteroid premedication, teplizumab was also tolerated at higher doses to prevent T-cell–mediated allograft rejection in patients with renal transplant.7 Whether the antileukemic activity of teplizumab and/or foralumab observed in the current report will be retained at clinically tolerated doses in T-ALL patients remains to be evaluated in phase 1/2 studies.
The antileukemic effect of induced TCR/CD3 signaling in T-ALL comes in stark contrast to mature T-cell neoplasms, such as peripheral T-cell lymphoma, where TCR signaling is thought to promote survival and oncogenesis. Therefore, the selective pressure of an anti-CD3 antibody may favor outgrowth of leukemic clones with negative or dim expression of CD3. Indeed, the authors detected such a selection of CD3-negative clones in some T-ALL PDX treated with anti-CD3 antibodies.1,2 Although antigen escape may limit the efficacy of the CD3-specific monotherapy, the authors demonstrated that a combinatorial approach with chemotherapy further suppresses propagation of resistant leukemic clones.1 Future studies should explore the efficacy of combining these antibodies with other therapeutic agents such as immunotherapies. Moreover, eliminating CD3+ T-ALL blasts may reduce tumor burden significantly enough to enable patients to proceed with potentially curative allogeneic stem cell transplant.
Unlike B-ALL, where targeting CD19, CD20, or CD22 with monoclonal antibodies and bispecific engagers can produce significant clinical benefit, options for immunotherapy of refractory and relapsed T-ALL remain very scarce, contributing to poor prognosis.8 The aggressive nature and rapid progression of this disease require agents that are fast and effective. Recent studies have demonstrated the efficacy of a cytotoxic anti-CD38 antibody daratumumab in PDX-based preclinical models of T-ALL9 ; a phase 2 clinical trial in pediatric and young adult patients with lymphoblastic leukemia is currently underway (#NCT03384654). In addition, chimeric antigen receptor T-cell–based therapies targeting CD5 and CD7 are being evaluated in phase 1 studies in patients with T-ALL.10 This work by Tran Quang and colleagues reveals CD3 as a very promising target for the immunotherapy of medullary T-ALL, a much-needed potential therapeutic option for patients with refractory or relapsed disease.
Conflict-of-interest disclosure: The author declares no competing financial interests.