Key Points
The composite of recurrent VTE and major bleeding was nonsignificantly lower for DOACs than for LMWHs.
DOACs should be used with caution in patients at high risk for bleeding.
Abstract
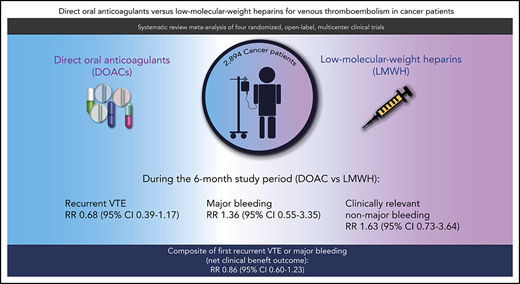
Direct oral anticoagulants (DOACs) are an emerging treatment option for patients with cancer and acute venous thromboembolism (VTE), but studies have reported inconsistent results. This systematic review and meta-analysis compared the efficacy and safety of DOACs and low-molecular-weight heparins (LMWHs) in these patients. MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, and conference proceedings were searched to identify relevant randomized controlled trials. Additional data were obtained from the original authors to homogenize definitions for all study outcomes. The primary efficacy and safety outcomes were recurrent VTE and major bleeding, respectively. Other outcomes included the composite of recurrent VTE and major bleeding, clinically relevant nonmajor bleeding (CRNMB), and all-cause mortality. Summary relative risks (RRs) were calculated in a random effects meta-analysis. In the primary analysis comprising 2607 patients, the risk of recurrent VTE was nonsignificantly lower with DOACs than with LMWHs (RR, 0.68; 95% CI, 0.39-1.17). Conversely, the risks of major bleeding (RR, 1.36; 95% CI, 0.55-3.35) and CRNMB (RR, 1.63; 95% CI, 0.73-3.64) were nonsignificantly higher. The risk of the composite of recurrent VTE or major bleeding was nonsignificantly lower with DOACs than with LMWHs (RR, 0.86; 95% CI, 0.60-1.23). Mortality was comparable in both groups (RR, 0.96; 95% CI, 0.68-1.36). Findings were consistent during the on-treatment period and in those with incidental VTE. In conclusion, DOACs are an effective treatment option for patients with cancer and acute VTE, although caution is needed in patients at high risk of bleeding.
Introduction
Venous thromboembolism (VTE) is a common complication in patients with cancer. Its management in these patients is challenging because the risks of bleeding events and recurrent VTE during anticoagulant treatment are high.1 Until recently, subcutaneous low-molecular-weight heparin (LMWH) was the mainstay of treatment for cancer-associated VTE, because of the lower risk of recurrent VTE compared with vitamin K antagonists.2,3 However, LMWH is relatively expensive, and the subcutaneous route of administration is often considered burdensome by patients, possibly leading to poor adherence.4-6
Direct oral anticoagulants (DOACs) include apixaban, edoxaban, and rivaroxaban, which inhibit factor Xa, and dabigatran, which inhibits thrombin. These drugs are easy to use because they can be administered orally in fixed doses with no routine monitoring. Based on their favorable safety profile compared with that of vitamin K antagonists, DOACs have been established as the recommended treatment of VTE in the general population.7
Recently, several randomized controlled trials have compared DOACs with LMWHs for the treatment of VTE in patients with cancer-associated VTE.8-11 Some of these trials showed that DOACs, compared with LMWHs, were associated with a higher risk of bleeding,8,9 while others did not.10,11 These conflicting results could reflect differences in patient characteristics across the studies (ie, index VTE, type and stage of cancer), heterogeneity in the definition of study outcomes, or variability in follow-up period analyses (ie, complete follow-up period vs on-treatment period). In addition, most studies were designed as noninferiority studies and therefore had limited precision in evaluating the efficacy or safety of DOACs relative to LMWHs. Hence, questions remain about the overall benefit–risk ratio of DOACs vs LMWHs for VTE treatment in patients with cancer.
The current systematic review and meta-analysis evaluated the efficacy and safety of DOACs vs LMWHs for the treatment of VTE in patients with cancer. In addition, by collecting additional outcome data for the individual studies, this report extends current knowledge by: (1) aggregating all current evidence using a uniform study outcome definition; (2) reporting a summary of the on-treatment analysis; (3) providing a subgroup analysis of patients with incidental VTE; and (4) performing an analysis of net clinical benefit by evaluating the composite outcome of first recurrent VTE and major bleeding.
Methods
This study was registered at Open Science Framework (https://osf.io/vn9aq). The study report was prepared in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidance (supplemental Table 1, available on the Blood Web site).12
Search strategy and data collection
A systematic literature search was performed on 29 March 2020, in MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials to identify randomized controlled trials that compared any DOAC with any LMWH for the treatment of VTE in patients with cancer by combining terms for VTE, deep vein thrombosis (DVT), pulmonary embolism (PE), cancer, and DOACs. No restrictions for year of publication or language were applied. The search strategy is shown in supplemental Table 2. In addition, a manual search was performed to identify relevant abstracts presented at the most recent conferences of the American Society of Hematology, the International Society on Thrombosis and Haemostasis (ISTH), the European Society of Cardiology, and the American College of Cardiology. All included studies were approved by their local institutional review board.
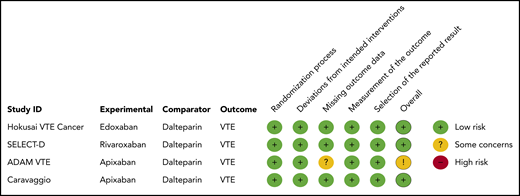
Two reviewers (F.I.M. and F.T.M.B.) independently screened titles, abstracts, and subsequently full texts for potentially eligible articles. Both reviewers independently assessed risk of bias by using the Cochrane risk-of-bias tool in randomized controlled trials version 2.0.13 Bias was assessed in the domains of randomization process, deviations from intended intervention, missing outcome data, measurement of outcome, and selection of the reported results. Data on study characteristics and outcomes were extracted by using a standardized form. Discrepancies were resolved by consensus or contact with a third reviewer (N.v.E.).
Inclusion criteria and outcomes
Randomized controlled trials that compared a therapeutic dose of a DOAC vs a therapeutic dose of an LMWH (either full dose or full dose followed by reduced dose) in patients with acute symptomatic or incidental VTE and active cancer or a recent history of cancer were eligible. Active cancer was defined as any cancer other than non-melanoma skin cancer that: (1) was diagnosed in the 6 months before study inclusion; (2) required cancer treatment in the 6 months before randomization; (3) was recurrent or metastatic; or (4) was a hematologic malignancy not in complete remission. A recent history of cancer was defined as a cancer diagnosis in the 2 years before inclusion and not fulfilling the criteria for active cancer. Any objectively confirmed symptomatic or incidental VTE was allowed as an index venous thromboembolic event, except superficial vein thrombosis.
The primary efficacy outcome was recurrent VTE, defined as symptomatic or incidental new DVT of the upper or lower extremities, symptomatic or incidental PE involving segmental or more proximal pulmonary arteries, or fatal PE including unexplained death for which PE could not be ruled out. Splanchnic vein thrombosis, cerebral vein thrombosis, and arterial thromboembolic events were not part of the primary efficacy outcome.
The primary safety outcome was major bleeding, defined by using the ISTH criteria as overt bleeding associated with a drop in hemoglobin of ≥2 g/dL or transfusion of ≥2 units of blood, or that occurred in a critical site or contributed to death.14 Other safety outcomes were all-cause mortality, fatal recurrent VTE, fatal major bleeding, and clinically relevant nonmajor bleeding, which was defined by using the ISTH criteria as overt bleeding not meeting the criteria for major bleeding but associated with the need for medical intervention, contact with a physician, interruption of the assigned treatment, discomfort, or impairment of activities of daily living.14 The definitions of fatal PE and major bleeding used in the original studies were accepted. The composite outcome of first recurrent VTE or major bleeding was also evaluated as a measure of net clinical benefit.
We intended to evaluate all outcomes during a maximum of 6 months of follow-up in the intention-to-treat or modified intention-to-treat population, which included all patients who were randomized to treatment and received at least 1 dose of the assigned study drug. Recurrent VTE and major bleeding events were also evaluated during the on-treatment period (while taking the assigned study drug or up to 3 days after discontinuation) in the per-protocol population as defined in the individual studies. If the per-protocol population was not specified, the (modified) intention-to-treat population was used for the on-treatment analysis. The certainty of evidence was graded according to the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) guidelines and presented in a summary of findings with GRADEpro GDT software.12
Statistical analysis
The primary effect measure was the relative risk (RR) for DOACs compared with LMWHs for all study outcomes. The secondary effect measure was the absolute risk reduction for DOACs relative to LMWH. Because RRs are generally more consistent across studies than absolute risks,15 the anticipated absolute risk reduction was calculated by applying the summary estimate of the RR to the observed baseline risk in the LMWH groups, as is generally recommended.15,16
Logit transformation and inverse variance weighting was used to calculate summary estimates using a Knapp-Hartung random effects model, which is generally preferred in a meta-analysis of few studies.17,18 Forest plots were constructed for all back-transformed outcome measures with corresponding 95% confidence intervals (CIs). Between-study heterogeneity was assessed by calculating τ2 and I2 using restricted maximum likelihood estimations.
The primary analysis was restricted to studies that included patients with acute, symptomatic, or incidentally detected lower-extremity DVT involving the popliteal, femoral, or iliac veins or the inferior vena cava, acute symptomatic PE, or incidentally detected PE involving segmental or more proximal pulmonary arteries as the index event. A secondary analysis included results from studies that had enrolled patients with any type of VTE index event.
A subgroup analysis was performed for patients whose index event was incidental VTE, which was defined as VTE events detected on radiologic imaging scans performed for reasons other than suspected VTE. Of the incidental VTE in the Hokusai VTE Cancer Study, only those confirmed by blinded adjudication were included.19
Publication bias was explored by visual inspection of the funnel plots. No formal tests for publication bias were performed, as they lack statistical power when only a few studies are included. Additional data were requested from the corresponding authors because there were differences across studies in outcome definitions and the reporting of on-treatment analyses, the composite outcome, and the subgroups gastrointestinal cancer and incidental index VTE.
Analyses were performed with R computing software version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org), using the meta package version 4.9-2.
Results
The electronic database and manual searches for conference abstracts yielded 545 unique articles and 1 abstract; 215 were duplicates, and 318 were excluded after screening of title and abstract. After full-text assessment, another 9 studies were excluded because the study was either a duplicate (n = 3), not a randomized trial (n = 2), included a different population (n = 3), or did not evaluate a DOAC (n = 1) (supplemental Figure 1). Interobserver agreement for study selection was 97% (Cohen’s κ = 0.72).
Four randomized controlled trials that had enrolled a total of 2894 patients with cancer and acute VTE were included in the meta-analysis.8-11 Study characteristics are specified in Table 1. In all studies, the proportion of patients with active cancer was ≥97%. The included studies evaluated either apixaban 5 mg twice daily (ADAM-VTE [Apixaban, Dalteparin, in Active Cancer Associated Venous Thromboembolism] and CARAVAGGIO),10,11 edoxaban 60 mg once daily (Hokusai VTE Cancer),8 or rivaroxaban 20 mg once daily (SELECT-D [Anticoagulation Therapy in Selected Cancer Patients at Risk of Recurrence of Venous Thromboembolism]).9 The patients in the control groups in all studies received subcutaneous dalteparin (200 IU/kg for the first 30 days, followed by 150 IU/kg thereafter). One study included upper-extremity and splanchnic DVT as index events,10 whereas the others limited inclusion to patients with proximal DVT or PE. All studies allowed inclusion of patients with incidental VTE. Two studies followed up patients for 6 months10,11 and two for 12 months.8,9
Baseline characteristics and 6-month study outcomes of included randomized trials
| Study name . | Year . | Treatment allocation* . | Baseline characteristics . | Study outcomes . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male . | Age (mean/median with SD/IQR), y . | Index event PE ± DVT . | Incidental VTE . | Prior VTE . | Metastatic disease¶ . | Gastro-intestinal cancer . | Recurrent VTE† . | Major bleeding . | Clinically relevant nonmajor bleeding . | All-cause mortality . | |||
| Hokusai VTE Cancer | 2018 | Edoxaban, n = 522‡ | 277 (53.1) | 64 ± 11 | 328 (62.8) | 167 (32.0) | 49 (9.4) | 274 (52.5) | 165 (31.6) | 34 (6.5) | 29 (5.6) | 64 (12.3) | 140 (26.8) |
| Dalteparin, n = 524 | 263 (50.2) | 63 ± 12 | 329 (62.8) | 173 (33.0) | 63 (12.0) | 280 (53.4) | 140 (26.7) | 46 (8.8) | 17 (3.2) | 43 (8.2) | 127 (24.2) | ||
| Select-D | 2018 | Rivaroxaban, n = 203 | 116 (57.1) | 67 (22-87) | 150 (73.9) | 108 (53.2) | NR | 118 (58.1) | 94 (46.3) | 7 (3.4) | 11 (5.4) | 25 (12.3) | 48 (23.6) |
| Dalteparin, n = 203 | 98 (48.3) | 67 (34-87) | 145 (71.4) | 105 (51.7) | NR | 118 (58.1) | 86 (42.4) | 17 (8.4) | 6 (3.0) | 7 (3.5) | 56 (27.6) | ||
| ADAM-VTE§ | 2020 | Apixaban, n = 150 | 72 (48.0) | 64 ± 11 | 81 (54.0) | NR | 8 (5.3) | 96 (64.0) | 48 (32.0) | 0 (0) | 0 (0) | 9 (6.2) | 23 (15.9) |
| Dalteparin, n = 150 | 73 (48.7) | 64 ± 11 | 75 (50.0) | NR | 12 (8.0) | 97 (64.7) | 57 (38.0) | 5 (3.5) | 2 (1.4) | 7 (4.9) | 15 (10.6) | ||
| CARAVAGGIO | 2020 | Apixaban, n = 576 | 292 (50.7) | 67 ± 11 | 304 (52.8) | 116 (20.1) | 45 (7.8) | 389 (67.5) | 188 (32.6) | 32 (5.6) | 22 (3.8) | 52 (9.0) | 135 (23.4) |
| Dalteparin, n = 579 | 276 (47.7) | 67 ± 11 | 334 (57.7) | 114 (19.7) | 61 (10.5) | 396 (68.4) | 187 (32.3) | 46 (7.9) | 23 (4.0) | 35 (6.0) | 153 (26.4) | ||
| Study name . | Year . | Treatment allocation* . | Baseline characteristics . | Study outcomes . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male . | Age (mean/median with SD/IQR), y . | Index event PE ± DVT . | Incidental VTE . | Prior VTE . | Metastatic disease¶ . | Gastro-intestinal cancer . | Recurrent VTE† . | Major bleeding . | Clinically relevant nonmajor bleeding . | All-cause mortality . | |||
| Hokusai VTE Cancer | 2018 | Edoxaban, n = 522‡ | 277 (53.1) | 64 ± 11 | 328 (62.8) | 167 (32.0) | 49 (9.4) | 274 (52.5) | 165 (31.6) | 34 (6.5) | 29 (5.6) | 64 (12.3) | 140 (26.8) |
| Dalteparin, n = 524 | 263 (50.2) | 63 ± 12 | 329 (62.8) | 173 (33.0) | 63 (12.0) | 280 (53.4) | 140 (26.7) | 46 (8.8) | 17 (3.2) | 43 (8.2) | 127 (24.2) | ||
| Select-D | 2018 | Rivaroxaban, n = 203 | 116 (57.1) | 67 (22-87) | 150 (73.9) | 108 (53.2) | NR | 118 (58.1) | 94 (46.3) | 7 (3.4) | 11 (5.4) | 25 (12.3) | 48 (23.6) |
| Dalteparin, n = 203 | 98 (48.3) | 67 (34-87) | 145 (71.4) | 105 (51.7) | NR | 118 (58.1) | 86 (42.4) | 17 (8.4) | 6 (3.0) | 7 (3.5) | 56 (27.6) | ||
| ADAM-VTE§ | 2020 | Apixaban, n = 150 | 72 (48.0) | 64 ± 11 | 81 (54.0) | NR | 8 (5.3) | 96 (64.0) | 48 (32.0) | 0 (0) | 0 (0) | 9 (6.2) | 23 (15.9) |
| Dalteparin, n = 150 | 73 (48.7) | 64 ± 11 | 75 (50.0) | NR | 12 (8.0) | 97 (64.7) | 57 (38.0) | 5 (3.5) | 2 (1.4) | 7 (4.9) | 15 (10.6) | ||
| CARAVAGGIO | 2020 | Apixaban, n = 576 | 292 (50.7) | 67 ± 11 | 304 (52.8) | 116 (20.1) | 45 (7.8) | 389 (67.5) | 188 (32.6) | 32 (5.6) | 22 (3.8) | 52 (9.0) | 135 (23.4) |
| Dalteparin, n = 579 | 276 (47.7) | 67 ± 11 | 334 (57.7) | 114 (19.7) | 61 (10.5) | 396 (68.4) | 187 (32.3) | 46 (7.9) | 23 (4.0) | 35 (6.0) | 153 (26.4) | ||
Data are expressed as No. (%) unless otherwise indicated. IQR, interquartile range; NR, not reported; SD, standard deviation.
Number of patients in (modified) intention-to-treat analysis.
Excluding splanchnic vein thrombosis and cerebral thrombosis.
A total of 122 (23.4%) patients receiving edoxaban met the criteria for dose reduction to 30 mg edoxaban once daily.
In the ADAM-VTE trial, baseline characteristics were presented for all 300 randomized patients, and the analysis was performed in the modified intention-to-treat population (n = 145 in the rivaroxaban group, n = 142 in the dalteparin group).
For the CARAVAGGIO study, this number also includes patients with recurrent locally advanced cancer.
Additional unpublished data were obtained for 3 studies to homogenize definitions and results,8-10 including the study outcome recurrent VTE without splanchnic or cerebral vein thrombosis,9,10 major gastrointestinal bleeding,8 outcomes for patients with incidental VTE,9 bleeding events during the on-treatment period according to our definition of bleeding up until 3 days after study drug discontinuation,8-10 bleeding events in the overall study period,10 and the net clinical benefit outcome as determined by the composite of first recurrent VTE or major bleeding.9,10
Risk of bias assessment
Using the Cochrane risk-of-bias tool in randomized controlled trials version 2.0, none of the studies was judged to be at high risk of bias for one of the bias domains. Despite the open-label study design, all studies were considered to be at low risk of bias in the domain “measurement of the outcome” because outcomes were centrally adjudicated by committees blinded to treatment allocation. One study was assessed as having “some concerns” in the bias domain of missing data, because 5% of patients were lost to follow-up.10 The results of the risk-of-bias assessment are presented in Figure 1. Visual inspection of the funnel plot did not indicate evidence of publication bias (supplemental Figure 2).
Primary analysis
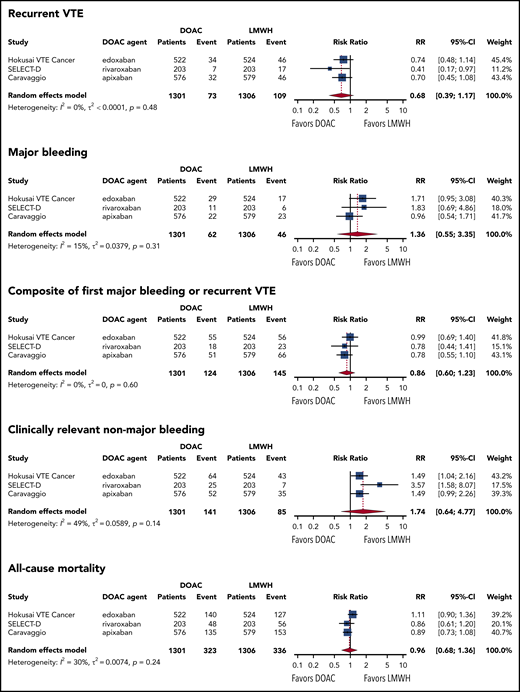
In the 3 studies that restricted enrollment to patients with proximal DVT or PE (N = 2607),8,9,11 the risk of recurrent VTE was nonsignificantly lower in the DOAC group than in the LMWH group (RR, 0.68; 95% CI, 0.39 to 1.17; I2 = 0%; moderate-quality evidence). With a baseline risk of recurrent VTE of 8.3% in LMWH users, the absolute risk reduction with DOACs was −2.7% (95% CI, −5.1 to 1.4). Conversely, the risk of major bleeding was nonsignificantly higher in the DOAC group than in the LMWH group during the follow-up period (RR, 1.36; 95% CI, 0.55 to 3.35; I2 = 15%; moderate-quality evidence). With a baseline risk of major bleeding of 3.5% in LMWH users, the absolute risk increase with DOACs was 1.3% (95% CI, −1.6 to 8.3). The net clinical benefit composite of first recurrent VTE or major bleeding was nonsignificantly lower in the DOAC group than in the LMWH group (RR, 0.86; 95% CI, 0.60 to 1.23; I2 = 0%; moderate-quality evidence), with an absolute risk reduction with DOACs of −1.6% (95% CI, –4.4 to 2.6). The risk of clinically relevant nonmajor bleeding was also nonsignificantly higher in the DOAC group than in the LMWH group (RR, 1.63; 95% CI, 0.73 to 3.64; I2 = 49%; moderate-quality evidence). Based on a baseline risk of clinically relevant nonmajor bleeding of 6.5% in LMWH users, the absolute risk increase with DOAC was 4.1% (95% CI, −1.8 to 17.2). All-cause mortality was comparable in both treatment groups (RR, 0.96; 95% CI, 0.68 to 1.36; I2 = 30%; moderate-quality of evidence). Figure 2 shows the forest plots of the RR of these study outcomes in both treatment groups. The risk of fatal VTE was nonsignificantly higher in the DOAC group than in the LMWH group (RR, 1.25; 95% CI, 0.26 to 5.94) (supplemental Figure 3). Conversely, the risk of fatal bleeding was nonsignificantly lower in the DOAC group than in the LMWH group (RR, 0.37; 95% CI, 0.03 to 3.91). The summary of findings, including absolute risk reduction and quality of evidence according to the GRADE criteria, is presented in Table 2.
Forest plots of summary RRs between the DOAC and LMWH groups. Results are based on the primary analysis, which included 3 studies that used PE or proximal DVT as the index event. For CARAVAGGIO, bleeding events during the on-treatment period were used.
Forest plots of summary RRs between the DOAC and LMWH groups. Results are based on the primary analysis, which included 3 studies that used PE or proximal DVT as the index event. For CARAVAGGIO, bleeding events during the on-treatment period were used.
Summary of findings for DOACs vs LMWHs for the treatment of cancer-associated thrombosis
| Outcome . | No. of participants (studies) . | Certainty of the evidence (GRADE) . | RR (95% CI) . | Observed risk with LMWH . | Anticipated absolute effects . | |
|---|---|---|---|---|---|---|
| Risk with DOACs* . | Absolute risk difference . | |||||
| Recurrent VTE | 2607 (3 RCTs) | ⊕⊕⊕◯ MODERATE due to imprecision† | 0.68 (0.39 to 1.17) | 8.3% | 5.6% | −2.7% (–5.1 to 1.4) |
| Major bleeding | 2607 (3 RCTs) | ⊕⊕⊕◯ MODERATE due to imprecision† | 1.36 (0.55 to 3.35) | 3.5% | 4.8% | 1.3% (–1.6 to 8.3) |
| Composite outcome of first recurrent VTE and major bleeding | 2607 (3 RCTs) | ⊕⊕⊕◯ MODERATE due to imprecision† | 0.86 (0.60 to 1.23) | 11.1% | 9.5% | −1.6% (–4.4 to 2.6) |
| Clinically relevant nonmajor bleeding | 2607 (3 RCTs) | ⊕⊕⊕◯ MODERATE due to imprecision† | 1.63 (0.73 to 3.64) | 6.5% | 10.6% | 4.1% (–1.8 to 17.2) |
| All-cause mortality | 2607 (3 RCTs) | ⊕⊕⊕◯ MODERATE due to imprecision† | 0.96 (0.68 to 1.36) | 25.7% | 24.7% | −1.0% (–8.2 to 9.3) |
| On-treatment analyses | ||||||
| Recurrent VTE (on-treatment) | 2440 (3 RCTs) | ⊕⊕⊕⊕ HIGH | 0.60 (0.38 to 0.95) | 8.1% | 4.9% | −3.2% (–5.0 to −0.4) |
| Major bleeding (on-treatment) | 2440 (3 RCTs) | ⊕⊕⊕◯ MODERATE due to imprecision† | 1.43 (0.46 to 4.45) | 3.2% | 4.6% | 1.4% (–1.7 to 11.0) |
| Clinically relevant nonmajor bleeding (on-treatment) | 2440 (3 RCTs) | ⊕⊕⊕◯ MODERATE due to imprecision† | 1.93 (0.70 to 5.31) | 4.6% | 8.9% | 4.3% (–1.4 to 19.7) |
| Outcome . | No. of participants (studies) . | Certainty of the evidence (GRADE) . | RR (95% CI) . | Observed risk with LMWH . | Anticipated absolute effects . | |
|---|---|---|---|---|---|---|
| Risk with DOACs* . | Absolute risk difference . | |||||
| Recurrent VTE | 2607 (3 RCTs) | ⊕⊕⊕◯ MODERATE due to imprecision† | 0.68 (0.39 to 1.17) | 8.3% | 5.6% | −2.7% (–5.1 to 1.4) |
| Major bleeding | 2607 (3 RCTs) | ⊕⊕⊕◯ MODERATE due to imprecision† | 1.36 (0.55 to 3.35) | 3.5% | 4.8% | 1.3% (–1.6 to 8.3) |
| Composite outcome of first recurrent VTE and major bleeding | 2607 (3 RCTs) | ⊕⊕⊕◯ MODERATE due to imprecision† | 0.86 (0.60 to 1.23) | 11.1% | 9.5% | −1.6% (–4.4 to 2.6) |
| Clinically relevant nonmajor bleeding | 2607 (3 RCTs) | ⊕⊕⊕◯ MODERATE due to imprecision† | 1.63 (0.73 to 3.64) | 6.5% | 10.6% | 4.1% (–1.8 to 17.2) |
| All-cause mortality | 2607 (3 RCTs) | ⊕⊕⊕◯ MODERATE due to imprecision† | 0.96 (0.68 to 1.36) | 25.7% | 24.7% | −1.0% (–8.2 to 9.3) |
| On-treatment analyses | ||||||
| Recurrent VTE (on-treatment) | 2440 (3 RCTs) | ⊕⊕⊕⊕ HIGH | 0.60 (0.38 to 0.95) | 8.1% | 4.9% | −3.2% (–5.0 to −0.4) |
| Major bleeding (on-treatment) | 2440 (3 RCTs) | ⊕⊕⊕◯ MODERATE due to imprecision† | 1.43 (0.46 to 4.45) | 3.2% | 4.6% | 1.4% (–1.7 to 11.0) |
| Clinically relevant nonmajor bleeding (on-treatment) | 2440 (3 RCTs) | ⊕⊕⊕◯ MODERATE due to imprecision† | 1.93 (0.70 to 5.31) | 4.6% | 8.9% | 4.3% (–1.4 to 19.7) |
GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate (the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different). Low certainty: our confidence in the effect estimate is limited (the true effect may be substantially different from the estimate of the effect). Very low certainty: we have very little confidence in the effect estimate (the true effect is likely to be substantially different from the estimate of effect). RCTs, randomized controlled trials.
The risk in the DOAC group (and its 95% CI) is based on the observed risk in the LMWH group and the relative effect of the intervention (and its 95% CI).
Graded down for imprecision due to a broad 95% CIs with a possible positive and negative effect of DOAC vs LMWH.
During the on-treatment period, the risk of recurrent VTE was significantly lower in the DOAC group compared with the LMWH group (RR, 0.60; 95% CI, 0.38 to 0.95; I2 = 0%; high-quality evidence). The risk of major bleeding and clinically relevant nonmajor bleeding was not materially different than in the total observation period. RRs of recurrent VTE, major bleeding, and clinically relevant nonmajor bleeding during the on-treatment period are shown in Table 2 and supplemental Figure 4.
Subgroup with incidental VTE
The primary analysis in patients with incidental VTE as their index event was based on data from 774 patients with cancer enrolled in 3 trials.8,9,11 Among patients with incidental VTE, the risk of recurrent VTE was nonsignificantly lower in the DOAC group than in the LMWH group (RR, 0.54; 95% CI, 0.26 to 1.11; I2 = 0%; moderate-quality evidence). With a baseline risk of recurrence in the LMWH group of 6.4%, the absolute risk reduction with a DOAC was −2.9% (95% CI, −4.7 to 0.7). The risk of major bleeding was nonsignificantly higher in patients with incidental VTE receiving DOACs than in those receiving LMWHs (RR, 1.29%; 95% CI, 0.74 to 2.28; I2 = 0%; moderate-quality evidence). With a baseline risk of major bleeding in the LMWH group of 4.6%, the absolute risk increase with DOACs was 1.3% (95% CI, −1.2 to 5.9). There was no evidence of interaction between type of index VTE (symptomatic vs incidental VTE) and treatment of the study outcomes recurrent VTE (P = .1677) or major bleeding (P = .5225). The summary of findings for this subgroup in the primary analysis is shown in Table 3, and the corresponding forest plot of the RR is given in supplemental Figure 5.
Summary of findings for DOACs vs LMWHs for the treatment of cancer-associated thrombosis in patients with incidental VTE
| Outcome . | No. of participants (studies) . | Certainty of the evidence (GRADE) . | RR (95% CI) . | Observed risk with LMWH . | Anticipated absolute effects . | |
|---|---|---|---|---|---|---|
| Risk with DOACs* . | Absolute risk difference . | |||||
| Recurrent VTE | 774 (3 RCTs) | ⊕⊕⊕◯ MODERATE due to imprecision† | 0.54 (0.26 to 1.11) | 6.4% | 3.5% | −2.9% (–4.7 to 0.7) |
| Major bleeding | 774 (3 RCTs) | ⊕⊕⊕◯ MODERATE due to imprecision† | 1.29 (0.74 to 2.28) | 4.6% | 5.9% | 1.3% (–1.2 to 5.9) |
| Outcome . | No. of participants (studies) . | Certainty of the evidence (GRADE) . | RR (95% CI) . | Observed risk with LMWH . | Anticipated absolute effects . | |
|---|---|---|---|---|---|---|
| Risk with DOACs* . | Absolute risk difference . | |||||
| Recurrent VTE | 774 (3 RCTs) | ⊕⊕⊕◯ MODERATE due to imprecision† | 0.54 (0.26 to 1.11) | 6.4% | 3.5% | −2.9% (–4.7 to 0.7) |
| Major bleeding | 774 (3 RCTs) | ⊕⊕⊕◯ MODERATE due to imprecision† | 1.29 (0.74 to 2.28) | 4.6% | 5.9% | 1.3% (–1.2 to 5.9) |
GRADE Working Group grades of evidence. High certainty; we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate (the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different). Low certainty: our confidence in the effect estimate is limited (the true effect may be substantially different from the estimate of the effect). Very low certainty: we have very little confidence in the effect estimate (the true effect is likely to be substantially different from the estimate of effect). RCTs, randomized controlled trials.
The risk in the DOAC group (and its 95% CI) is based on the observed risk in the LMWH group and the relative effect of the intervention (and its 95% CI).
Graded down for imprecision due to a broad 95% CI with a possible positive and negative effect of DOAC vs LMWH.
Secondary analysis
The secondary analysis was based on data from 2894 patients with cancer and any acute VTE index event from 4 trials.8-11 Overall, the results were comparable to those of the primary analysis. The risk of recurrent VTE was nonsignificantly lower in the DOAC group (RR, 0.66; 95% CI, 0.39 to 1.13; I2 = 26%; moderate-quality of evidence), whereas the risks of major bleeding (RR, 1.32; 95% CI, 0.70 to 2.47; I2 = 1%; moderate-quality of evidence) and clinically relevant nonmajor bleeding (RR, 1.60; 95% CI, 0.99 to 2.60; I2 = 29%; moderate-quality of evidence) were nonsignificantly higher. The risk of the composite of first recurrent VTE or major bleeding was nonsignificantly lower in the DOAC group than in the LMWH group (RR, 0.84; 95% CI, 0.63 to 1.34; I2 = 38%; moderate quality of evidence). All-cause mortality risk was similar in both groups (RR, 0.99; 95% CI, 0.74 to 1.32; I2 = 37%; moderate-quality evidence) (supplemental Figure 6; supplemental Table 3). No fatal events were reported in the ADAM-VTE trial.
In the on-treatment period, the risk of recurrent VTE was significantly lower in the DOAC group (RR, 0.59; 95% CI, 0.35 to 0.99; I2 = 5%; high-quality evidence), whereas the risk of major bleeding was nonsignificantly higher (RR, 1.35; 95% CI, 0.54 to 3.37; I2 = 36%; moderate-quality evidence). The risk of clinically relevant nonmajor bleeding in this period was significantly higher in DOAC recipients (RR, 1.83; 95% CI, 1.06 to 3.14; I2 = 18%; high-quality evidence) (supplemental Figure 7; supplemental Table 3). The ADAM-VTE trial did not report incidental index events, and therefore this subgroup could not be evaluated in the secondary analysis.
Discussion
In this systematic review and meta-analysis, data from 4 randomized controlled trials that compared DOACs with LMWHs for cancer-associated VTE were aggregated. The main finding is that, compared with LMWHs, DOACs were associated with a 32% lower risk of recurrent VTE and a 36% higher risk of major bleeding, although these effects were not statistically significant. The net clinical benefit, defined as the composite of recurrent VTE and major bleeding, was nonsignificantly lower in the DOAC group. Results did not change materially when only considering events during treatment with the study drug.
The results of this meta-analysis support DOACs as an acceptable treatment option for cancer-associated VTE, thereby strengthening current guidelines.20-22 Results in patients with incidentally detected VTE, a group that is rapidly expanding because of the widespread use of high-resolution computed tomography scanning for staging and follow-up of cancer patients, were consistent with those in patients with symptomatic events.
Compared with LMWH, the reported risk of major bleeding and clinically relevant nonmajor bleeding was nonsignificantly higher with DOACs than with LMWHs, suggesting that DOACs should be avoided in patients at high risk of bleeding. One such group includes patients with gastrointestinal cancer, as the majority of major bleeding events occurred in the gastrointestinal tract (36 [58%] of 62 events). An increased risk of major bleeding in patients with gastrointestinal cancer was observed in the Hokusai VTE Cancer and SELECT-D studies9,23,24 but not in the ADAM-VTE study.10 Despite these observations, the incidence of major bleeding in this patient group was not reported in the later CARAVAGGIO study.11 However, the overall major bleeding risk was not increased in apixaban recipients despite that one-third (33%) of these patients had gastrointestinal cancer. It is unclear whether these conflicting results between studies relate to the pharmacodynamic parameters of the particular DOAC (apixaban vs others). Although head-to-head comparisons between DOACs in the setting of atrial fibrillation or VTE are lacking, observational studies suggest that some DOACs may be associated with a lower risk of gastrointestinal bleeding than others.25-27 Another potential explanation could be the enrollment of fewer gastrointestinal cancer patients at high risk for bleeding in the CARAVAGGIO and ADAM-VTE studies, which were conducted after publication of the Hokusai VTE Cancer and SELECT-D study results.
Overall, only a few fatal VTE and major bleeding events were reported in the studies (range, 0%-0.6%), resulting in relatively wide CIs around the summary estimates. The risk of fatal VTE in each study may have been underestimated because adjudication of fatal VTE is often difficult in the cancer population when detailed information on death is not available.28 Case fatality rates for VTE were 6.8% in the DOAC group and 3.4% in the LMWH group, whereas case fatality rates for major bleeding were 1.6% and 10.4% in these groups, respectively. The apparent higher case fatality rate with LMWHs could reflect a more severe course of bleeding than with DOACs, which has been reported previously.24
The majority of patients in the trials received systemic cancer therapy. Plasma concentrations of DOACs can be altered by drugs that inhibit or induce P-glycoprotein or cytochrome P-450 3A4, including several chemotherapeutic agents, tyrosine kinase inhibitors, tamoxifen, and immunomodulating agents such as dexamethasone.29 Unfortunately, we were unable to evaluate such potential interactions because data on this subgroup of patients were not available. More research is needed to better understand the pharmacokinetic parameters of DOACs when given concurrently with cancer drugs.
All trials in this meta-analysis had included a broad spectrum of cancer patients, and the proportion of patients with metastatic disease was consistent and substantial, ranging from 53% to 65%. Nonetheless, important differences between the 4 individual trials included in this meta-analysis should be noted. The risk of major bleeding in the Hokusai VTE Cancer, SELECT-D, and CARAVAGGIO studies ranged from 4% to 6% in the DOAC group, and from 3% to 4% in the LMWH group. In ADAM-VTE, which was not included in the primary analysis, there were no bleeding events in the DOAC group and only 2 in the LMWH group (1.4%). Possible explanations include differences in case-mix as reflected by the substantially lower mortality risk in ADAM-VTE or because patients with upper-extremity DVT and splanchnic vein thrombosis were enrolled in ADAM-VTE but not in the other studies. The secondary analysis, however, which included the ADAM-VTE study, did not yield materially different results.
Strengths of this systematic review and meta-analysis include the availability of additional data from 3 studies, which allowed us to homogenize study outcomes, evaluate additional outcomes, and perform subgroup analyses for patients with incidental VTE. Between-study heterogeneity in the analyses was generally low and most of the included studies were considered to be at low risk of bias in all the bias domains. The study population comprised 2894 patients with cancer, the majority of whom received cancer treatment and had metastatic disease, making the study groups representative of clinical practice. Because only 4 studies were included in this study, the Knapp-Hartung method was used to combine results, which less likely leads to artificially small CIs.17 Our estimates of uncertainty, as reflected by the CIs, may therefore be more conservative than with other methods. However, the more commonly used DerSimonian-Laird method for random effects leads to false-positive findings in up to 15% of pooled results when <5 studies are included.30
Several limitations need to be acknowledged. Lack of detailed descriptions of patient characteristics precluded evaluation of several other important subgroups of patients, such as those with gastrointestinal, urogenital, or hematologic cancers. Precision in the individual trials and in this systematic review was limited for evaluating effects in the subgroup of patients with incidental VTE. Findings in this subgroup should therefore be interpreted with caution. All included studies were performed with an open-label design because subcutaneous placebo injections were considered inappropriate. The risk of bias in outcome assessment was considered low because there was central adjudication of study outcomes with blinding of allocated treatment in all included trials.
The present findings show that DOACs are an effective treatment option and are safe for most patients with cancer and acute VTE. DOACs should be used with caution in patients at high risk for bleeding. Choosing the optimal anticoagulant drug for cancer-associated VTE should be based on a careful balance of the risks of recurrent VTE and bleeding, the consideration of potential drug–drug interactions, and patient preference.
For original data, please contact the corresponding author (Frits I. Mulder; e-mail: f.i.mulder@amsterdamumc.nl).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: F.I.M. and F.T.M.B. performed the data extraction, analyses, and wrote the first draft; and all authors made a substantial contribution to the concept and design of the study, interpreted the data, reviewed the manuscript, critically revised the paper for important intellectual content, approved the final version, and agree with the submission.
Conflict-of-interest disclosure: A.M.Y. received research funding from Bayer; and honoraria from Bayer, BMS/Pfizer Alliance, and Leo Pharma. A.M. received research funding from Bayer. M.C. received research funding from LEO Pharma, Bristol-Myers Squibb, and Pfizer; and advisory board honoraria from Bayer, Bristol-Myers Squibb, LEO Pharma, Pfizer, Servier, and Sanofi. P.W.K. received research funding from Daiichi Sankyo and Roche Diagnostics. J.I.W. declares consultancy and receipt of honoraria from Anthos, Bayer AG, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Ionis, Janssen, Merck, Novartis, Pfizer, PhaseBio, Portola, Servier, and Tetherex Pharmaceuticals; and institutional grants from Bayer AG and Boehringer Ingelheim. S.M. received grants and fees paid to her institution from GlaxoSmithKline, BMS/Pfizer, Aspen, Daiichi Sankyo, Bayer, Boehringer Ingelheim, Sanofi, and Portola. N.v.E. reports advisory board honoraria from Daiichi-Sankyo, LEO Pharma, and Bayer. The remaining authors declare no competing financial interests.
Correspondence: Frits I. Mulder, Department of Vascular Medicine, Amsterdam Cardiovascular Science, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: f.i.mulder@amsterdamumc.nl.
REFERENCES
Author notes
F.I.M. and F.T.M.B. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal