In this issue of Blood, Bhatlekar et al1 have identified microRNA (miRNA) miR-125-5p as a regulator of L-plastin, an actin bundling protein, and show how L-plastin levels control platelet formation by megakaryocytes.
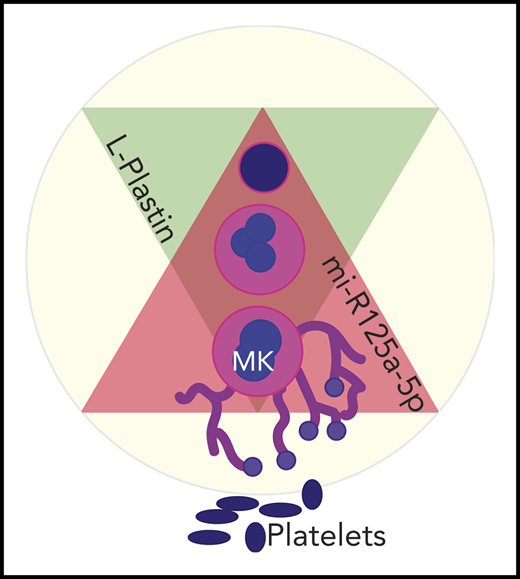
Negative association between L-plastin and human platelet numbers proposed by Bhatlekar et al in this issue of Blood. Levels of miR-125a-5p (magenta triangle) increase during megakaryopoiesis. miR-125a-5p targets and downregulates expression of L-plastin (lime green triangle), thus enhancing proplatelet formation by megakaryocytes (MK).
Negative association between L-plastin and human platelet numbers proposed by Bhatlekar et al in this issue of Blood. Levels of miR-125a-5p (magenta triangle) increase during megakaryopoiesis. miR-125a-5p targets and downregulates expression of L-plastin (lime green triangle), thus enhancing proplatelet formation by megakaryocytes (MK).
When blood vessels are damaged, blood loss has to be stopped. This is the function of small blood cells called platelets. These cells form aggregates and create a plug that sticks to the wound to prevent bleeding. You want to have the right amount of this platelet “glue” though, since too few or too many platelets give rise to conditions such as thrombocytopenia and thrombocytosis, which increase the risk of serious bleeding and clot formation, respectively. Platelet production is an intriguing process. It starts in the bone marrow, where the platelet progenitor cells, called megakaryocytes, develop and increase in size during their maturation process. Finally, numerous platelets are released from each megakaryocyte via the formation of elongated branches, called proplatelets, in a process that requires extreme changes to the cell’s structure and cytoplasmic organization. These changes in cell shape and motility depend on the actin cytoskeleton. The importance of the cytoskeleton in platelet biogenesis is illustrated by the reduction in platelet formation in conditions characterized by mutations of cytoskeletal components such as tubulin2 and myosin.3 Whether changes in the cytoskeleton can increase platelet formation is not known. Gaining a better understanding of this aspect of platelet formation is of substantial clinical and physiological importance.
Bhatlekar et al, in this issue of Blood, identified miRNA miR-125a-5p as a positive regulator of platelet production by skillfully correlating their previous findings on miRNAs associated with platelet count in 154 healthy donors4 with miRNA profiling in (i) purified mature bone marrow megakaryocytes, (ii) platelets from the same bone marrow donors, and (iii) megakaryocytes derived from cord blood cells in the laboratory. Following a similar rigorous approach, the authors narrowed down the thousands of potential miR-125a-5p targets and identified L-plastin as one of the main factors downregulated by miR-125a-5p in megakaryocytes. L-plastin (LCP1) is an actin-binding protein that links actin filaments and stabilizes parallel strands. This paper shows that lower levels of L-plastin facilitate platelet formation (see figure).
The authors first established that knockdown of miR-125a-5p led to lower platelet formation. Conversely, overexpression of miR-125a-5p boosted platelet numbers. They then confirmed the inverse relationship between miR-125a-5p and L-plastin expression by increasing and decreasing miR-125a-5p levels in human megakaryocytes and mouse bone marrow. To understand the role of L-plastin in platelet production, Bhatlekar et al used CRISPR-Cas9 gene editing technology to lower L-plastin levels and show that this reduction considerably increased proplatelet formation and proplatelet branching (which positively correlates with platelet formation). Megakaryocytes require actin-containing structures called podosomes to degrade extracellular matrix, which is necessary for delivery of platelets into the circulation.5 In this study, the authors demonstrate that L-plastin knockdown markedly increased the number of podosomes per megakaryocyte. This is likely to contribute to enhanced platelet release in vivo. Together, these observations provide evidence of a new nexus in platelet production characterized by the inverse relationship between miR-125a-5p and L-plastin.
Of course, there are interesting remaining questions. The authors observed a stronger phenotype upon manipulation of miR-125a-5p than following targeting of L-plastin. Presumably, miR-125a-5p downregulates additional targets in megakaryocytes that, coincidentally, also lead to increased platelet production. How L-plastin regulates podosome development, whether increased podosome numbers are relevant for in vitro proplatelet formation, and the function of L-plastin in the megakaryocyte invaginated membrane system are yet to be determined. Development of megakaryocyte-specific miR-125a-5p and/or L-plastin knockout murine models would be invaluable, since conflicting results have been reported in existing global knockout and overexpression models.6,7
These findings have potential implications for disorders of platelet number, since dysregulation of the miR-125a-5p/L-plastin axis could be a factor in the pathogenesis of these conditions. In this paper, the authors present proof of principle that judicious manipulation of miR-125a-5p could be used to balance abnormal platelet numbers. In addition, the long-term ambition of producing sufficient platelets in vitro for autotransfusion would benefit from strategies that might optimize platelet yield per megakaryocyte, such as overexpression of miR-125a-5p or knockdown of L-plastin. Overall, the authors offer a novel insight into the captivating process that fragments megakaryocytes, the largest cells in bone marrow, into the smallest cells in the circulation.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal