Key Points
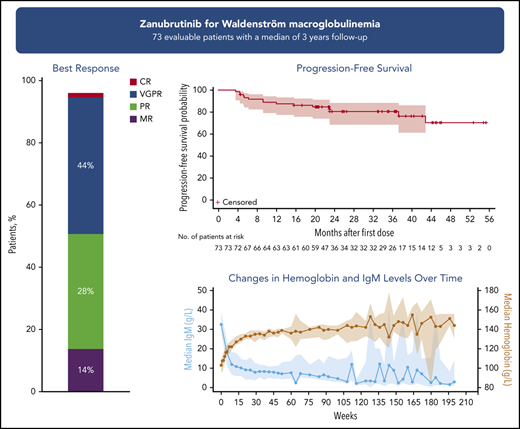
Long-term zanubrutinib treatment of patients with WM resulted in an overall response rate of 96% and a VGPR/CR rate of 45%.
Long-term treatment with single-agent zanubrutinib was well tolerated in both treatment-naïve and relapsed/refractory patients.
Abstract
Inhibitors of Bruton’s tyrosine kinase (BTK) have established therapeutic activity in patients with Waldenström macroglobulinemia (WM). Zanubrutinib, a potent and selective BTK inhibitor, was evaluated in a phase 1/2 study in patients with WM who were either treatment-naïve (TN) or had relapsed/refractory (R/R) disease. Patients had disease requiring treatment per International Workshop on Waldenström Macroglobulinemia (IWWM) criteria. Treatment was 160 mg of oral zanubrutinib twice daily (n = 50) or 320 mg once daily (n = 23). Efficacy endpoints included overall response rate (ORR) and very good partial response/complete response (VGPR/CR) rates per IWWM-6 criteria (with modification of VGPR definition published previously). Between September 2014 and March 2018, 77 patients (24 TN and 53 R/R) began treatment. At a median follow-up of 36.0 months for patients with R/R disease and 23.5 months for TN, 72.7% remained on treatment. Reasons for treatment discontinuation included any adverse events in 13.0% of patients (1 treatment related), disease progression (10.4%), and other (3.9%). The ORR was 95.9%, and the VGPR/CR rate was 45.2%, which increased over time: 20.5% at 6 months, 32.9% at 12 months, and 43.8% at 24 months. Estimated 3-year progression-free survival rate was 80.5%, and overall survival rate was 84.8%. Adverse events of interest included contusion (32.5%, all grade 1), neutropenia (18.2%), major hemorrhage (3.9%), atrial fibrillation/flutter (5.2%), and grade 3 diarrhea (2.6%). Long-term treatment with single-agent zanubrutinib resulted in deep and durable responses in some patients with WM. The safety profile of long-term zanubrutinib therapy in these patients was acceptable. This trial was registered at www.clinicaltrials.gov as #NCT02343120.
Introduction
Waldenström macroglobulinemia (WM) is a mature B-cell neoplasm characterized by the growth and accumulation of lymphoplasmacytic lymphoma cells and secretion of monoclonal immunoglobulin M (IgM) by malignant cells.1 Most cases involve the bone marrow, and some involve lymph nodes and extranodal sites. Most patients experience weakness and fatigue from anemia. The monoclonal IgM protein can result in hyperviscosity syndrome, peripheral neuropathy, cryoglobulinemia, and immune complex vasculitis.2 Although clinically indolent, WM is incurable, and the disease course frequently includes episodes of symptomatic recurrence requiring treatment.
Molecularly, the disease is characterized by a specific point mutation in the MYD88 gene (MYD88L265P), present in over 90% of cases, which results in constitutive NF-κB activation.3 In ∼30% of patients, the malignant cells also have an additional mutation in the CXCR4 gene (CXCR4WHIM), encoding a chemokine receptor involved in cell–cell adhesion.4 Patients whose WM is wild type (WT) for MYD88 demonstrate significantly higher mortality than patients with WM harboring MYD88L265P.5 Data suggest that progression-free survival (PFS) and very good partial response (VGPR) rates are lower when patients with both MYD88L265P and CXCR4WHIM WM (compared with MYD88L265P alone) are treated with the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib.6
Inhibitors of BTK have established therapeutic activity in patients with WM. Ibrutinib, the first-in-class BTK inhibitor, was evaluated in 63 patients with relapsed or refractory (R/R) WM in a phase 2 study.7 With a median of 47 months of follow-up, the major response rate (MRR) was 78%, and median PFS was >5 years.8 In a companion study in 30 treatment-naïve (TN) patients with MYD88MUT disease, after a median of 13.4 months, the MRR was 83% and the VGPR rate was 20%.9 In both studies, MRRs were higher among patients with MYD88MUT/CXCR4WT disease than among patients who had MYD88MUT/CXCR4MUT disease. Patients with MYD88WT disease had the least favorable outcomes, with no major responses observed and a median PFS of 21 months.8
Patients with R/R WM treated with ibrutinib experienced toxicities, including infections (68%), diarrhea (42%), grade 1 or 2 bleeding (39%), increased tendency to bruise (23%), hypertension (23%), neutropenia (20%), thrombocytopenia (16%), fatigue (13%), and pneumonia (6%).10 Among patients with R/R WM, 11% developed atrial fibrillation, 6% discontinued ibrutinib for toxicity, and 3% had disease transformation.11 It is believed that inhibition of structurally related tyrosine kinases such as epidermal growth factor receptor (EGFR), tyrosine kinase expressed in hepatocellular carcinoma, interleukin-2–inducible T-cell kinase, and others12 may explain many of the toxicities, including diarrhea, bleeding,13,14 and atrial fibrillation.15
Zanubrutinib is a novel, potent, and selective BTK inhibitor. In kinase inhibition and cell-based assays, it was more selective than ibrutinib for BTK inhibition, exhibiting less off-target activity against EGFR, tyrosine kinase expressed in hepatocellular carcinoma, interleukin-2–inducible T-cell kinase, and other tyrosine kinases.16 Zanubrutinib is maximally absorbed in 2 to 3 hours, with a 2- to 4-hour terminal half-life.12 At the recommended phase 2 dose (RP2D) of 160 mg twice daily, zanubrutinib steady-state plasma levels were approximately eightfold higher than those observed for ibrutinib at 560 mg daily, resulting in complete and sustained BTK inhibition in blood and lymph node compartments.12,17,18
In early clinical studies, zanubrutinib was well tolerated, demonstrating promising antitumor activity in a variety of mature B-cell neoplasms, including WM, mantle cell lymphoma (MCL) and chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).12,19 Sustained complete (>95%) BTK occupancy in patient lymph node biopsy specimens was more frequent with 160 mg twice daily than 320 mg once daily (89% vs 50%; P = .034).12 Consequently, 160 mg twice daily was selected for further investigation. In a cohort of 78 patients with CLL/SLL treated with zanubrutinib monotherapy, the overall response rate (ORR) was 96.2% (95% confidence interval [95% CI], 89.2-99.2), and the estimated PFS at 12 months was 100%.12 In 86 patients with R/R MCL, after a median follow-up of 18.4 months, 84% achieved an objective response, with 68.6% achieving a complete response (CR). Median duration of response (DOR) and PFS were 19.5 and 22.1 months, respectively.20 In this study, we report the safety and efficacy of long-term zanubrutinib therapy in patients with TN and R/R WM, a subset of patients in an ongoing phase 1/2 study of B-cell malignancies.12
Methods
Study design and treatment
BGB-3111 AU-003 (NCT02343120) is a first-in-human, multicenter, phase 1/2 study of zanubrutinib in patients with B-cell malignancies at 24 sites in 6 countries. The study has 2 parts: part 1 is the dose-escalation portion focused on identifying the RP2D; part 2 is the expansion portion that includes several disease-specific patient cohorts. No maximally tolerated dose was identified in part 1, and both 160 mg twice daily or 320 mg daily were identified as the RP2Ds.12 Part 2 included mostly disease-specific patient cohorts with R/R B-cell malignancies and a smaller number of patients with TN MCL, CLL/SLL, and WM who either refused or were deemed unsuitable for standard frontline therapy were also enrolled. As data accumulated, 160 mg twice daily was eventually chosen as the preferred RP2D, based primarily on pharmacodynamic results demonstrating near complete BTK occupancy in disease-affected lymph nodes.12 The sample size determination for the WM cohort in part 2 was based on the assumption of a response rate of 80%. With a total of 50 patients enrolled, the lower boundary of the 95% CI was 66% if the observed response rate was 80%. Data presented in this article report outcomes for patients with TN or R/R WM enrolled in either part of the study.
All patients provided written informed consent. The study was conducted in accordance with the principles of the Declaration of Helsinki and International Conference on Harmonization guidelines. Institutional review boards and independent ethics committees approved the protocol. Data were collected by the investigators and their research teams. All authors had full access to and were responsible for analyzing and interpreting the data.
Patients
Eligible patients were required to be ≥18 years old; have Eastern Cooperative Oncology Group performance status 0 to 2; have adequate baseline hematologic (neutrophil and platelet counts >1.0 × 109/L and ≥50 × 109/L, respectively), renal (measured or estimated creatinine clearance ≥30 mL/min), and liver (transaminase levels ≤3 times the upper limit of normal, total bilirubin ≤1.5 times the upper limit of normal) function; and have no previous exposure to a BTK inhibitor. Patients with current central nervous system involvement, significant cardiac disease, histologic transformation to aggressive lymphoma, and those requiring concurrent, strong CYP3A inhibitors/inducers or QT-prolonging medications were excluded. Patients with a history of atrial fibrillation and those requiring concurrent antithrombotic medications (eg, aspirin, anticoagulants) were not excluded. Full inclusion/exclusion criteria are shown in the supplemental Methods, available on the Blood Web site.
Assessments
Blood samples for nephelometric IgM and paraprotein quantitation by serum protein electrophoresis (SPEP) were collected at screening, every 4 weeks through week 52, every 12 weeks thereafter, and at treatment discontinuation. Responses were investigator assessed in accordance with the 6th International Workshop on WM with modification for VGPR definition per Treon et al (supplemental Methods).21,22 If baseline quantitative IgM levels were unavailable, responses were assessed by changes in paraprotein levels measured by SPEP. All patients had bone marrow aspiration and biopsy at screening, within 7 days of the end of week 12, and thereafter as clinically indicated for confirmation of CR or disease progression. All patients had computed tomography (CT) scans at baseline; those with extramedullary disease (lymphadenopathy or splenomegaly by imaging or physician exam, as described in the supplemental Methods) had CT scans every 12 weeks until week 48 and every 24 weeks thereafter. Quality of life data were not collected in this study.
Safety assessments included monitoring of type, frequency, severity, and outcomes of adverse events (AEs). All AEs that occurred from the first treatment day until 30 days after study treatment discontinuation were summarized. AEs of interest were a predefined subgroup of AEs known to be associated with ibrutinib. They were identified in accordance with predefined Medical Dictionary for Regulatory Activities, version 22.0, search criteria and included bleeding (including major hemorrhage), atrial fibrillation/flutter, hypertension, second primary malignancies (including skin cancers), tumor lysis syndrome, infections (including opportunistic infections), neutropenia, thrombocytopenia, and anemia. AE severity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
MYD88 mutational status was analyzed on bone marrow aspirates using a proprietary, polymerase chain reaction–based assay that uses locked oligonucleotides to block amplification of MYD88WT DNA during polymerase chain reaction followed by bidirectional Sanger sequencing of the amplicon.23 This approach captures all mutations from amino acids 260 to 278 in the Toll/IL-1R domain of MYD88 with limit of detection ∼0.5%. Mutations in CXCR4 were detected using a validated next-generation sequencing assay (supplemental Methods). Samples were collected at baseline where possible, but if unavailable, postbaseline samples were assessed for MYD88/CXCR4 genotype. B-cell selection was not used for MYD88 or CXCR4 determination.
Statistical analyses
Safety analyses included all patients with WM receiving at least 1 zanubrutinib dose and no prior BTK inhibitor exposure (a later cohort enrolled 1 patient with WM intolerant to prior BTK inhibitor therapy; this patient was excluded from the analysis). Standard descriptive statistics were used to summarize AE data in the safety population. The efficacy-evaluable population consisted of all patients in the safety population with a baseline IgM level ≥5 g/L. The primary efficacy endpoint was the proportion of patients achieving VGPR or CR. This endpoint was based on the observation that patients achieving a VGPR after treatment with rituximab-based chemoimmunotherapy had a PFS outcome similar to patients achieving a CR.24 Other efficacy endpoints included MRR (greater than or equal to PR), ORR (greater than or equal to minimal response), PFS, DOR, overall survival (OS), and changes from baseline in serum IgM levels, hemoglobin concentrations, and extramedullary disease burden. Rates and depths of response as a function of MYD88/CXCR4 genotype were also examined.
Response rates were summarized as the percentage of responders for each category (CR and VGPR, MRR, and ORR) with 95% CIs.25 DOR was assessed as the time from first qualifying response until disease progression or death from any cause. The proportion of patients with VGPR/CR over time was estimated with simple proportions (number of patients in VGPR/CR up to each time point divided by the total number of patients). PFS was measured from the time of first study drug dose to disease progression or death from any cause. Patients not experiencing progressive disease (PD) or death were censored on the day of last tumor assessment before subsequent anticancer therapy initiation for DOR and PFS analyses. Median DOR, PFS and event-free rates at landmark time points were estimated using Kaplan-Meier methodology with corresponding 95% CIs.26 Median follow-up times for PFS and DOR were estimated by the reverse Kaplan-Meier method. OS was defined as time from first study drug dose until death from any cause.
Results
Treatment and patient disposition
Between September 2014 and March 2018, 77 patients with WM (24 TN and 53 R/R) and without prior BTK inhibitor exposure were enrolled then treated with zanubrutinib. Seventy-three of these patients received an initial total daily dose of 320 mg. Of patients with R/R disease, 36 received 160 mg twice daily and 13 received 320 mg daily. Of patients with TN disease, 14 received 160 mg twice daily and 10 received 320 mg daily. Four patients with R/R disease received a starting dose of zanubrutinib <320 mg/day in the dose-escalation part of the study; of these, 3 patients escalated to a dose of 160 mg twice daily. Upon protocol amendment, 12 patients (4 R/R, 8 TN) who were initially assigned to 320 mg once daily were switched to 160 twice daily.
In the safety population, the median study follow-up was 36.0 months for patients with R/R disease and 23.5 months for TN patients, the difference in follow-up time between patients with R/R disease and TN patients was a result of exclusive enrollment of those with R/R disease at the beginning of the study. Fifty-six patients were continuing study treatment at the data cutoff date of August 31, 2019 (19 of 24 TN, 37 of 53 R/R). Twenty-one patients discontinued study drug: 10 patients for AEs (3 TN, 7 R/R), 8 patients for PD (1 TN, 7 R/R), 2 patients for other reasons (both R/R), and 1 patient for an investigator’s decision (1 TN) (supplemental Figure 1).
Patient demographics and baseline disease characteristics
Baseline characteristics are presented in Table 1. Median age was 67 years (range, 40-87 years), with approximately one-fifth of patients >75 years old. The majority were male. The median times from initial diagnosis to enrollment on study were 0.7 and 5.3 years for TN and R/R cohorts, respectively. Among the patients with R/R disease, the median number of prior regimens was 2 (range, 1-8). The most common prior treatments included rituximab-based therapy, alkylating agents, and corticosteroids (supplemental Table 1). Across both cohorts, approximately one-third of patients had serum IgM levels ≥40 g/L. Mutation testing was performed in 90% of patients (n = 69). Fifty-eight patients (20 TN, 38 R/R) had disease with a MYD88L265P mutation (84% of those tested); 11 (3 TN, 8 R/R) were MYD88WT. Among cases with MYD88L265P-mutated disease, WM in 11 patients also harbored CXCR4WHIM mutations (19%; 4 TN, 7 R/R). Among patients with MYD88WT disease, all 11 had CXCR4WT disease.
Demographic and clinical characteristics of patients at baseline
| Characteristic . | TN (n = 24) . | R/R (n = 53) . | Total (N = 77) . |
|---|---|---|---|
| Age | |||
| Years, median (range) | 65 (40-87) | 68 (45-87) | 67 (40-87) |
| >75 y, n (%) | 3 (12.5) | 13 (24.5) | 16 (20.8) |
| Male, n (%) | 16 (67) | 45 (85) | 61 (79) |
| ECOG performance status score, n (%) | |||
| 0/1 | 24 (100) | 50 (94) | 74 (96) |
| 2 | 0 (0) | 3 (6) | 3 (4) |
| Serum IgM (g/L)* | |||
| Median (range) | 43.9 (5.3-91.9) | 29.4 (1.2-88.5) | 32.4 (1.2-91.9) |
| ≥40 g/L, n (%) | 13 (54) | 11 (21) | 24 (31) |
| Baseline hemoglobin (g/L) | |||
| Median (range) | 100.5 (68-132) | 106.0 (63-155) | 105.0 (63-155) |
| ≤110 g/L, n (%) | 14 (58) | 32 (60) | 46 (60) |
| Extramedullary disease, n (%) | |||
| Lymphadenopathy† | 13 (54) | 26 (49) | 39 (51) |
| Splenomegaly | 9 (38) | 17 (32) | 26 (34) |
| Bone marrow infiltration, median (range) | |||
| Cellularity | 42.5 (10-95) | 27.5 (0-94) | 35 (0-95) |
| No. of prior systemic therapies | NA | 2 (1-8) | 2 (1-8) |
| Genotype, n (%)‡ | |||
| MYD88L265P/CXCR4WT | 14 (58.3) | 26 (49.1) | 40 (51.9) |
| MYD88L265P/CXCR4WHIM | 4 (16.7) | 7 (13.2) | 11 (14.3) |
| MYD88L265P/CXCR4FS | 2 (8.3) | 4 (7.5) | 6 (7.8) |
| MYD88L265P/CXCR4NS | 2 (8.3) | 3 (5.7) | 5 (6.5) |
| MYD88L265P/CXCR4UNK | 2 (8.3) | 5 (9.4) | 7 (9.1) |
| MYD88WT/CXCR4WT | 3 (12.5) | 8 (15.1) | 11 (14.3) |
| Characteristic . | TN (n = 24) . | R/R (n = 53) . | Total (N = 77) . |
|---|---|---|---|
| Age | |||
| Years, median (range) | 65 (40-87) | 68 (45-87) | 67 (40-87) |
| >75 y, n (%) | 3 (12.5) | 13 (24.5) | 16 (20.8) |
| Male, n (%) | 16 (67) | 45 (85) | 61 (79) |
| ECOG performance status score, n (%) | |||
| 0/1 | 24 (100) | 50 (94) | 74 (96) |
| 2 | 0 (0) | 3 (6) | 3 (4) |
| Serum IgM (g/L)* | |||
| Median (range) | 43.9 (5.3-91.9) | 29.4 (1.2-88.5) | 32.4 (1.2-91.9) |
| ≥40 g/L, n (%) | 13 (54) | 11 (21) | 24 (31) |
| Baseline hemoglobin (g/L) | |||
| Median (range) | 100.5 (68-132) | 106.0 (63-155) | 105.0 (63-155) |
| ≤110 g/L, n (%) | 14 (58) | 32 (60) | 46 (60) |
| Extramedullary disease, n (%) | |||
| Lymphadenopathy† | 13 (54) | 26 (49) | 39 (51) |
| Splenomegaly | 9 (38) | 17 (32) | 26 (34) |
| Bone marrow infiltration, median (range) | |||
| Cellularity | 42.5 (10-95) | 27.5 (0-94) | 35 (0-95) |
| No. of prior systemic therapies | NA | 2 (1-8) | 2 (1-8) |
| Genotype, n (%)‡ | |||
| MYD88L265P/CXCR4WT | 14 (58.3) | 26 (49.1) | 40 (51.9) |
| MYD88L265P/CXCR4WHIM | 4 (16.7) | 7 (13.2) | 11 (14.3) |
| MYD88L265P/CXCR4FS | 2 (8.3) | 4 (7.5) | 6 (7.8) |
| MYD88L265P/CXCR4NS | 2 (8.3) | 3 (5.7) | 5 (6.5) |
| MYD88L265P/CXCR4UNK | 2 (8.3) | 5 (9.4) | 7 (9.1) |
| MYD88WT/CXCR4WT | 3 (12.5) | 8 (15.1) | 11 (14.3) |
ECOG, Eastern Cooperative Oncology Group; FS, frameshift mutation; NA, not applicable; NS, nonsense mutation; UNK, unknown.
On the basis of nephelometric assessment (n = 74) or in the absence of a quantitative IgM level with SPEP.
Thirty-one patients had baseline lymphadenopathy on the basis of CT imaging alone.
Genotype data were obtained from baseline bone marrow aspirate samples, or if not available, postbaseline samples. Eight patients (1 TN and 7 R/R) did not provide bone marrow samples for MYD88/CXCR4 genomic profiling. Five of 8 patients had enrolled prior to protocol requirement for bone marrow analysis for MYD88/CXCR4, and 3 of 8 patients did not sign the optional informed consent for genetic testing of bone marrow.
Response rates
Seventy-three patients were evaluable for efficacy (another 4 had baseline IgM concentrations <5 g/L). Across both cohorts, 45.2% (95% CI, 33.5-57.3) of patients achieved a best response of VGPR or CR (33.3% [95% CI, 15.6-55.3] and 51.0% [95% CI, 36.3-65.6] in the TN and R/R cohorts, respectively; Table 2). Major responses were seen in 82.2% of patients (87.5% TN, 79.6% R/R). Median time to major response was 2.8 months for TN and R/R cohorts. The proportion of patients achieving a best response of VGPR/CR increased with treatment duration: 20.5% at 6 months, 32.9% at 12 months, and 43.8% at 24 months. In patients with R/R disease, the rate of VGPR/CR was 24.5% at 6 months, 38.8% at 12 months, and 49.0% at 24 months; evidence of a plateau began at ∼20 months (supplemental Figure 2). ORR, MRR, and VGPR/CR rates in the 160-mg twice-daily arm were 97.9%, 80.9%, and 48.9% as compared with the 320-mg daily arm at 90.9%, 81.8%, and 31.8% (supplemental Table 3).
Efficacy outcomes
| . | TN (n = 24) . | R/R (n = 49) . | Total (N = 73) . |
|---|---|---|---|
| Duration of follow-up, median, mo | 23.5 | 35.8 | 30.3 |
| Best overall response, n (%) | |||
| CR | 0 | 1 (2.0) | 1 (1.4) |
| VGPR | 8 (33.3) | 24 (49.0) | 32 (43.8) |
| PR | 13 (54.2) | 14 (28.6) | 27 (37.0) |
| MR | 3 (12.5) | 7 (14.3) | 10 (13.7) |
| SD | 0 | 3 (6.1) | 3 (4.1) |
| PD | 0 | 0 | 0 |
| VGPR/CR rate, % (95% CI) | 33.3 (15.6-55.3) | 51.0 (36.3-65.6) | 45.2 (33.5-57.3) |
| VGPR/CR rate by genotype, % (95% CI) | |||
| MYD88L265P/CXCR4WT (n = 39) | 59.0 (42.1-74.4) | ||
| MYD88L265P/CXCR4WHIM (n = 11) | 27.3 (6.0-61.0) | ||
| MYD88L265P/CXCR4FS (n = 6) | 33.3 (4.3-77.7) | ||
| MYD88L265P/CXCR4NS (n = 5) | 20.0 (0.5-71.6) | ||
| MYD88WT (n = 8) | 25.0 (3.2-65.1) | ||
| ORR (MR or better), % (95% CI) | 100.0 (85.8-100.0) | 93.9 (83.1-98.7) | 95.9 (88.5-99.1) |
| MRR (PR or better), % (95% CI) | 87.5 (67.6-97.3) | 79.6 (65.7-89.8) | 82.2 (71.5-90.2) |
| PFS estimate at 24 mo, % (95% CI) | 91.5 (70.0-97.8) | 76.2 (60.9-86.2)* | 80.5 (68.5-88.3) |
| OS estimated at 24 mo, % (95% CI) | 100 (NE-NE) | 91.5 (78.8-96.7)* | 94.1 (84.9-97.7) |
| . | TN (n = 24) . | R/R (n = 49) . | Total (N = 73) . |
|---|---|---|---|
| Duration of follow-up, median, mo | 23.5 | 35.8 | 30.3 |
| Best overall response, n (%) | |||
| CR | 0 | 1 (2.0) | 1 (1.4) |
| VGPR | 8 (33.3) | 24 (49.0) | 32 (43.8) |
| PR | 13 (54.2) | 14 (28.6) | 27 (37.0) |
| MR | 3 (12.5) | 7 (14.3) | 10 (13.7) |
| SD | 0 | 3 (6.1) | 3 (4.1) |
| PD | 0 | 0 | 0 |
| VGPR/CR rate, % (95% CI) | 33.3 (15.6-55.3) | 51.0 (36.3-65.6) | 45.2 (33.5-57.3) |
| VGPR/CR rate by genotype, % (95% CI) | |||
| MYD88L265P/CXCR4WT (n = 39) | 59.0 (42.1-74.4) | ||
| MYD88L265P/CXCR4WHIM (n = 11) | 27.3 (6.0-61.0) | ||
| MYD88L265P/CXCR4FS (n = 6) | 33.3 (4.3-77.7) | ||
| MYD88L265P/CXCR4NS (n = 5) | 20.0 (0.5-71.6) | ||
| MYD88WT (n = 8) | 25.0 (3.2-65.1) | ||
| ORR (MR or better), % (95% CI) | 100.0 (85.8-100.0) | 93.9 (83.1-98.7) | 95.9 (88.5-99.1) |
| MRR (PR or better), % (95% CI) | 87.5 (67.6-97.3) | 79.6 (65.7-89.8) | 82.2 (71.5-90.2) |
| PFS estimate at 24 mo, % (95% CI) | 91.5 (70.0-97.8) | 76.2 (60.9-86.2)* | 80.5 (68.5-88.3) |
| OS estimated at 24 mo, % (95% CI) | 100 (NE-NE) | 91.5 (78.8-96.7)* | 94.1 (84.9-97.7) |
Percentages are based on N, the number of patients who received ≥1 dose of zanubrutinib and had baseline IgM or M-paraprotein ≥5 g/L.
MR, minimal response; NE, not evaluable; PR, partial response; SD, stable disease.
For patients with R/R WM, 36-month PFS estimate was 76.2% (95% CI, 60.9-86.2) and 36-month OS estimate was 80.2 (63.8-89.7).
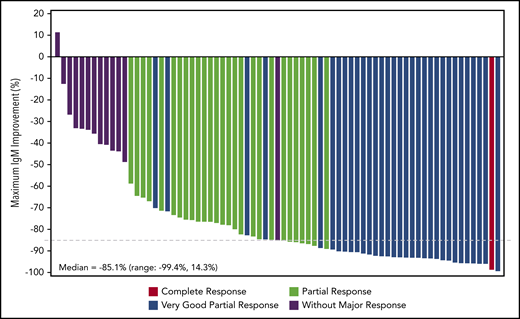
Among efficacy-evaluable patients with available genotype data (n = 65), the proportion with MYD88L265P mutated disease achieving VGPR/CR was 49.1% (28 of 57) and 25% (2 of 8) for those with MYD88WT WM (including 1 CR and 1 VGPR). The VGPR/CR rate in the subset of patients who had MYD88L265P/CXCR4WT WM was 59.0% (23 of 39), and 27.3% (3 of 11) in patients with MYD88L265P/CXCR4WHIM WM (Figure 1). However, MRRs were similar between patients with MYD88L265P/CXCR4WT and MYD88L265P/CXCR4WHIM genotypes overall (34 of 39 [87.2%] and 10 of 11 [90.9%], respectively). Median DORs were not reached for any response category (Table 2).
Best overall response by MYD88/CXCR4 genotype. For efficacy-evaluable patients with tumors harboring the MYD88L265P mutation, best response is reported separately for those with CXCR4WT disease and those with an accompanying CXCR4WHIM mutation. No genotype data were available for 8 patients. The CXCR4 genotype is unknown (CXCR4UNK) for an additional 7 patients with MYD88L265P disease.
Best overall response by MYD88/CXCR4 genotype. For efficacy-evaluable patients with tumors harboring the MYD88L265P mutation, best response is reported separately for those with CXCR4WT disease and those with an accompanying CXCR4WHIM mutation. No genotype data were available for 8 patients. The CXCR4 genotype is unknown (CXCR4UNK) for an additional 7 patients with MYD88L265P disease.
IgM, hemoglobin, and extramedullary disease
Serum IgM levels decreased with increasing treatment duration (Figure 2; supplemental Figure 3). Overall, 36 patients had dose holds of ≥8 days; 18 of whom experienced an IgM rebound of ≥50%. Of those 18 patients, 13 patients experienced subsequent IgM declines comparable to nadir levels, 3 patients had IgM declines but not to nadir levels, and 2 patients progressed.
Waterfall plot of maximal percentage of IgM reductions and corresponding best overall response. Patients included are those who received ≥1 dose of zanubrutinib, had no prior BTK inhibitor exposure, and had baseline IgM or M-paraprotein ≥5 g/L. Only patients with data at both baseline and any postbaseline visits are included. If the nephelometric IgM test result was missing at baseline, the M-paraprotein result by SPEP was used throughout and summarized together with nephelometric IgM test results for this endpoint.
Waterfall plot of maximal percentage of IgM reductions and corresponding best overall response. Patients included are those who received ≥1 dose of zanubrutinib, had no prior BTK inhibitor exposure, and had baseline IgM or M-paraprotein ≥5 g/L. Only patients with data at both baseline and any postbaseline visits are included. If the nephelometric IgM test result was missing at baseline, the M-paraprotein result by SPEP was used throughout and summarized together with nephelometric IgM test results for this endpoint.
Hemoglobin concentrations increased with time on treatment, exhibiting a median maximal improvement of 35 g/L, a 32.7% improvement over baseline (25th and 75th percentiles: 17.8%, 56.6%). Among patients with a baseline hemoglobin concentration ≤110 g/L, the median maximal increase was more pronounced at 44.5 g/L.
Among the 31 patients with baseline lymphadenopathy by CT scan, 16 (51.6%) exhibited >50% reduction in the sum of the products of perpendicular diameters of target lesions while on study. The median maximal reduction from baseline in target lymph node sum of the products of perpendicular diameters was 53.4%. Similarly, all 18 patients with both baseline splenomegaly and postbaseline spleen assessment exhibited reductions in craniocaudal spleen length measured by CT scan while on study; median maximal reduction was 19.2%. Follow-up bone marrow examinations were required only for patients with bone marrow involvement at baseline, and only at end of week 12. Among patients with serial bone marrow biopsies (20 TN, 41 R/R), there was no change in the median percentage of involvement by lymphoma at week 13 compared with baseline.
Survival
The median PFS in patients with R/R disease was not reached after a median follow-up of 36.8 months (Figure 3B). The estimated event-free rates at 18, 24, and 36 months were 83.7%, 76.2%, and 76.2%, respectively. OS for patients with R/R disease is shown in supplemental Figure 4. There were 13 PFS events in R/R patients: 9 patients experienced PD and 4 patients died without PD. The median PFS in TN patients was not reached after a median follow-up of 23 months (Figure 3A). The estimated event-free rates at 18 and 24 months were both 91.5%. Two TN patients experienced PD and neither died. A landmark analysis did not demonstrate significant differences in PFS by best response at 12 months between VGPR/CR vs PR/MR (supplemental Figure 5). Exploratory subgroup analyses of PFS by dosing schedule or genotype were also performed (supplemental Figure 6), with no clear associations observed.
Kaplan-Meier plots of PFS. PFS for TN (A) and R/R (B) patients is shown. Gray areas indicate 95% CIs.
Kaplan-Meier plots of PFS. PFS for TN (A) and R/R (B) patients is shown. Gray areas indicate 95% CIs.
Safety
All patients reported at least 1 AE of any grade; 58.4% reported at least 1 grade 3 or higher AE (Table 3). The most commonly reported AEs were upper respiratory tract infection (51.9%), contusion (32.5%), and cough (22.1%). Grade 3 or higher AEs reported in more than 1 patient were neutropenia (15.6%); anemia (9.1%); basal cell carcinoma and cellulitis (each 5.2%); hypertension and pneumonia (each 3.9%); and diarrhea, headache, fall, and actinic keratosis (each 2.6%). In total, 9 patients died while on study (all R/R). Two patients died because of PD and 2 patients died from unknown causes. Five patients had AEs leading to death: abdominal sepsis (day 1242), bacterial arthritis (day 887), Scedosporium infection (day 62), gastric adenocarcinoma, (day 526), and worsening bronchiectasis (day 121) (supplemental Table 4). Four of the 5 events were assessed as not related to study drug. The relation between death and the study drug was not assessed for the patient with bacterial arthritis. A summary of AEs by dosing schedule is shown in supplemental Table 5.
Treatment-emergent AEs
| Event term* . | Grade 1, n (%) . | Grade 2, n (%) . | Grade 3, n (%) . | Grade 4, n (%) . | Grade 5, n (%) . | All grade, n (%) . |
|---|---|---|---|---|---|---|
| Patients with ≥1 AE | 5 (6.5) | 27 (35.1) | 30 (39.0) | 10 (13.0) | 5 (6.5) | 77 (100.0) |
| Nonhematologic AEs | ||||||
| Upper respiratory tract infection | 1 (1.3) | 39 (50.6) | 0 | 0 | 0 | 40 (51.9) |
| Contusion | 25 (32.5) | 0 | 0 | 0 | 0 | 25 (32.5) |
| Cough | 16 (20.8) | 1 (1.3) | 0 | 0 | 0 | 17 (22.1) |
| Diarrhea | 8 (10.4) | 5 (6.5) | 2 (2.6) | 0 | 0 | 15 (19.5) |
| Urinary tract infection | 1 (1.3) | 13 (16.9) | 1 (1.3) | 0 | 0 | 15 (19.5) |
| Headache | 8 (10.4) | 4 (5.2) | 2 (2.6) | 0 | 0 | 14 (18.2) |
| Rash | 11 (14.3) | 2 (2.6) | 0 | 0 | 0 | 13 (16.9) |
| Hypertension | 1 (1.3) | 8 (10.4) | 3 (3.9) | 0 | 0 | 12 (15.6) |
| Constipation | 7 (9.1) | 5 (6.5) | 0 | 0 | 0 | 12 (15.6) |
| Back pain | 10 (13.0) | 2 (2.6) | 0 | 0 | 0 | 12 (15.6) |
| Fatigue | 11 (14.3) | 1 (1.3) | 0 | 0 | 0 | 12 (15.6) |
| Gastroesophageal reflux disease | 7 (9.1) | 4 (5.2) | 0 | 0 | 0 | 11 (14.3) |
| Nausea | 8 (10.4) | 3 (3.9) | 0 | 0 | 0 | 11 (14.3) |
| Cellulitis | 1 (1.3) | 5 (6.5) | 4 (5.2) | 0 | 0 | 10 (13.0) |
| Epistaxis | 9 (11.7) | 1 (1.3) | 0 | 0 | 0 | 10 (13.0) |
| Oropharyngeal pain | 8 (10.4) | 2 (2.6) | 0 | 0 | 0 | 10 (13.0) |
| Petechiae | 10 (13.0) | 0 | 0 | 0 | 0 | 10 (13.0) |
| Pruritus | 9 (11.7) | 1 (1.3) | 0 | 0 | 0 | 10 (13.0) |
| Basal cell carcinoma | 1 (1.3) | 4 (5.2) | 0 | 0 | 0 | 9 (11.7) |
| Arthralgia | 4 (5.2) | 4 (5.2) | 1 (1.3) | 0 | 0 | 9 (11.7) |
| Fall | 2 (2.6) | 4 (5.2) | 2 (2.6) | 0 | 0 | 8 (10.4) |
| Lower respiratory tract infection | 0 | 8 (10.4) | 0 | 0 | 0 | 8 (10.4) |
| Pneumonia | 0 | 1 (1.3) | 3 (3.9) | 0 | 0 | 4 (5.2) |
| Actinic keratosis | 1 (1.3) | 0 | 2 (2.6) | 0 | 0 | 3 (3.9) |
| Hematologic AEs | ||||||
| Neutropenia† | 0 | 2 (2.6) | 6 (7.8) | 6 (7.8) | 0 | 14 (18.2) |
| Anemia | 1 (1.3) | 3 (3.9) | 7 (9.1) | 0 | 0 | 11 (14.3) |
| Event term* . | Grade 1, n (%) . | Grade 2, n (%) . | Grade 3, n (%) . | Grade 4, n (%) . | Grade 5, n (%) . | All grade, n (%) . |
|---|---|---|---|---|---|---|
| Patients with ≥1 AE | 5 (6.5) | 27 (35.1) | 30 (39.0) | 10 (13.0) | 5 (6.5) | 77 (100.0) |
| Nonhematologic AEs | ||||||
| Upper respiratory tract infection | 1 (1.3) | 39 (50.6) | 0 | 0 | 0 | 40 (51.9) |
| Contusion | 25 (32.5) | 0 | 0 | 0 | 0 | 25 (32.5) |
| Cough | 16 (20.8) | 1 (1.3) | 0 | 0 | 0 | 17 (22.1) |
| Diarrhea | 8 (10.4) | 5 (6.5) | 2 (2.6) | 0 | 0 | 15 (19.5) |
| Urinary tract infection | 1 (1.3) | 13 (16.9) | 1 (1.3) | 0 | 0 | 15 (19.5) |
| Headache | 8 (10.4) | 4 (5.2) | 2 (2.6) | 0 | 0 | 14 (18.2) |
| Rash | 11 (14.3) | 2 (2.6) | 0 | 0 | 0 | 13 (16.9) |
| Hypertension | 1 (1.3) | 8 (10.4) | 3 (3.9) | 0 | 0 | 12 (15.6) |
| Constipation | 7 (9.1) | 5 (6.5) | 0 | 0 | 0 | 12 (15.6) |
| Back pain | 10 (13.0) | 2 (2.6) | 0 | 0 | 0 | 12 (15.6) |
| Fatigue | 11 (14.3) | 1 (1.3) | 0 | 0 | 0 | 12 (15.6) |
| Gastroesophageal reflux disease | 7 (9.1) | 4 (5.2) | 0 | 0 | 0 | 11 (14.3) |
| Nausea | 8 (10.4) | 3 (3.9) | 0 | 0 | 0 | 11 (14.3) |
| Cellulitis | 1 (1.3) | 5 (6.5) | 4 (5.2) | 0 | 0 | 10 (13.0) |
| Epistaxis | 9 (11.7) | 1 (1.3) | 0 | 0 | 0 | 10 (13.0) |
| Oropharyngeal pain | 8 (10.4) | 2 (2.6) | 0 | 0 | 0 | 10 (13.0) |
| Petechiae | 10 (13.0) | 0 | 0 | 0 | 0 | 10 (13.0) |
| Pruritus | 9 (11.7) | 1 (1.3) | 0 | 0 | 0 | 10 (13.0) |
| Basal cell carcinoma | 1 (1.3) | 4 (5.2) | 0 | 0 | 0 | 9 (11.7) |
| Arthralgia | 4 (5.2) | 4 (5.2) | 1 (1.3) | 0 | 0 | 9 (11.7) |
| Fall | 2 (2.6) | 4 (5.2) | 2 (2.6) | 0 | 0 | 8 (10.4) |
| Lower respiratory tract infection | 0 | 8 (10.4) | 0 | 0 | 0 | 8 (10.4) |
| Pneumonia | 0 | 1 (1.3) | 3 (3.9) | 0 | 0 | 4 (5.2) |
| Actinic keratosis | 1 (1.3) | 0 | 2 (2.6) | 0 | 0 | 3 (3.9) |
| Hematologic AEs | ||||||
| Neutropenia† | 0 | 2 (2.6) | 6 (7.8) | 6 (7.8) | 0 | 14 (18.2) |
| Anemia | 1 (1.3) | 3 (3.9) | 7 (9.1) | 0 | 0 | 11 (14.3) |
MedDRA, Medical Dictionary for Regulatory Activities.
Data are for treatment-emergent AEs in the 77 zanubrutinib-treated patients with WM included in the study. Listed events occurred in >10% of patients (in >2% of patients for grade ≥3) on or before the data cutoff date of August 31, 2019.
Includes the MedDRA-preferred terms neutropenia, neutrophil count decreased, and febrile neutropenia.
The most commonly reported AEs of special interest were infections; 90.9% of patients reported at least 1 infection of any grade (treatment-related in 15 of 70 patients with event) and 27.3% of patients reported at least 1 grade ≥3 infection (Table 4). The most common grade ≥3 infections were cellulitis and pneumonia (n = 4 and n = 3, respectively, all R/R). The exposure-adjusted incidence rate (EAIR) for grade 3 or higher infections was 1.12 events per 100 person-months. Fungal infections occurred in 4 patients with R/R disease; the events were bronchopulmonary aspergillosis (grade 2), cryptococcal meningitis (grade 3), esophageal candidiasis (grade 2), and disseminated Scedosporium infection (grade 5). All of these infections, except the disseminated Scedosporium infection, resolved with treatment, and the patients were able to continue zanubrutinib therapy. The patient with Scedosporium infection had a prior history of neck Scedosporium abscesses that had been treated, but while on this study the infection recurred, worsened, and ultimately was fatal. One patient, an 80-year-old Asian male with R/R WM, not receiving prophylactic antiviral therapy, developed hepatitis B reactivation without elevation in aspartate aminotransferase or alanine aminotransferase. This was successfully treated with lamivudine, and the patient continued zanubrutinib without interruption or further complication.
AEI
| Category of event . | Grade 1, n (%) . | Grade 2, n (%) . | Grade 3, n (%) . | Grade 4, n (%) . | Grade 5, n (%) . | All grade, n (%) . | EAIR (all severity grades), events per 100 person-months . |
|---|---|---|---|---|---|---|---|
| Patients with ≥1 AEI* | 7 (9.1) | 30 (39.0) | 25 (32.5) | 9 (11.7) | 4 (5.2) | 75 (97.4) | – |
| Bleeding | 40 (51.9) | 5 (6.5) | 3 (3.9) | 0 | 0 | 48 (62.3) | 4.49 |
| Major hemorrhage | 0 | 0 | 3 (3.9) | 0 | 0 | 3 (3.9) | 0.13 |
| Atrial fibrillation/ flutter | 1 (1.3) | 2 (2.6) | 1 (1.3) | 0 | 0 | 4 (5.2) | 0.19 |
| Hypertension | 1 (1.3) | 8 (10.4) | 3 (3.9) | 0 | 0 | 12 (15.6) | 0.61 |
| Second primary malignancies | 3 (3.9) | 6 (7.8) | 7 (9.1) | 2 (2.6) | 1 (1.3) | 19 (24.7) | 0.98 |
| Skin cancers | 2 (2.6) | 7 (9.1) | 4 (5.2) | 0 | 0 | 13 (16.9) | 0.66 |
| Tumor lysis syndrome | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Infections | 4 (5.2) | 45 (58.4) | 17 (22.1) | 1 (1.3) | 3 (3.9) | 70 (90.9) | 11.48 |
| Hepatitis B reactivation* | 1 (1.3) | 0 | 0 | 0 | 0 | 1 (1.3) | – |
| Anemia | 1 (1.3) | 3 (3.9) | 7 (9.1) | 0 | 0 | 11 (14.3) | 0.54 |
| Neutropenia | 0 | 2 (2.6) | 6 (7.8) | 6 (7.8) | 0 | 14 (18.2) | 0.73 |
| Thrombocytopenia | 3 (3.9) | 2 (2.6) | 1 (1.3) | 0 | 0 | 6 (7.8) | 0.28 |
| Category of event . | Grade 1, n (%) . | Grade 2, n (%) . | Grade 3, n (%) . | Grade 4, n (%) . | Grade 5, n (%) . | All grade, n (%) . | EAIR (all severity grades), events per 100 person-months . |
|---|---|---|---|---|---|---|---|
| Patients with ≥1 AEI* | 7 (9.1) | 30 (39.0) | 25 (32.5) | 9 (11.7) | 4 (5.2) | 75 (97.4) | – |
| Bleeding | 40 (51.9) | 5 (6.5) | 3 (3.9) | 0 | 0 | 48 (62.3) | 4.49 |
| Major hemorrhage | 0 | 0 | 3 (3.9) | 0 | 0 | 3 (3.9) | 0.13 |
| Atrial fibrillation/ flutter | 1 (1.3) | 2 (2.6) | 1 (1.3) | 0 | 0 | 4 (5.2) | 0.19 |
| Hypertension | 1 (1.3) | 8 (10.4) | 3 (3.9) | 0 | 0 | 12 (15.6) | 0.61 |
| Second primary malignancies | 3 (3.9) | 6 (7.8) | 7 (9.1) | 2 (2.6) | 1 (1.3) | 19 (24.7) | 0.98 |
| Skin cancers | 2 (2.6) | 7 (9.1) | 4 (5.2) | 0 | 0 | 13 (16.9) | 0.66 |
| Tumor lysis syndrome | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Infections | 4 (5.2) | 45 (58.4) | 17 (22.1) | 1 (1.3) | 3 (3.9) | 70 (90.9) | 11.48 |
| Hepatitis B reactivation* | 1 (1.3) | 0 | 0 | 0 | 0 | 1 (1.3) | – |
| Anemia | 1 (1.3) | 3 (3.9) | 7 (9.1) | 0 | 0 | 11 (14.3) | 0.54 |
| Neutropenia | 0 | 2 (2.6) | 6 (7.8) | 6 (7.8) | 0 | 14 (18.2) | 0.73 |
| Thrombocytopenia | 3 (3.9) | 2 (2.6) | 1 (1.3) | 0 | 0 | 6 (7.8) | 0.28 |
AEI, AEs of interest.
EAIR not calculated.
Thirteen (54.2%) TN and 35 (66.0%) patients with R/R disease reported at least 1 bruising or bleeding event, 45 of 48 (93.8%) of which were grade 1 or 2, most commonly contusion (32.5%), petechiae (13.0%), epistaxis (13.0%), hematuria (7.8%), purpura, and rectal hemorrhage (3.9% each). Three patients experienced grade 3 hemorrhages: purpura in a TN patient, hemothorax and melena occurred in a TN patient (the hemothorax occurred following thoracentesis), and hemorrhagic cystitis in a patient with R/R disease who had locally recurrent bladder cancer. The hemorrhagic cystitis event was managed with treatment interruption for 11 days. One patient required zanubrutinib discontinuation for bleeding (grade 3 purpura). The EAIR for any hemorrhage and major hemorrhage were 4.49 events per 100 person-months and 0.13 events per 100 person-months, respectively.
Atrial fibrillation was reported in 4 (5.2%) patients (1 TN, 3 R/R) for an EAIR of 0.19 events per 100 person-months; 1 patient had a prior history of atrial fibrillation. One atrial fibrillation event in a TN patient was grade 3, and no patients required dose reductions or treatment discontinuation. Second primary malignancies (EAIR, 0.98 events per 100 person-months) consisted primarily of nonmelanoma skin cancers (ie, basal cell carcinoma, squamous cell carcinoma, intraepidermal squamous cell carcinoma; EAIR, 0.66 events per 100 patient-months), all but 1 in patients with R/R disease, and most from Australia and New Zealand, a region with particularly high background prevalence. Grade 3 or higher neutropenia was reported in 15.6% of patients. Ten patients received 1 to 8 courses of granulocyte colony stimulating factor. No patients required dose reduction or treatment discontinuation for neutropenia. No patient developed tumor lysis syndrome.
Discussion
The first-in-class BTK inhibitor, ibrutinib has demonstrated substantial activity in patients with WM. However, challenges remain with respect to the benefit/risk ratio of ibrutinib, especially for patients with disease of MYD88L265P/CXCR4WHIM and MYD88WT where response rates are lower, and for those experiencing toxicity. For this reason, we investigated zanubrutinib, a potent and selective BTK inhibitor, in patients with WM. Results from this ongoing study demonstrate that long-term treatment with zanubrutinib was tolerable and resulted in deep and durable responses in the majority of patients with WM.
After an overall median follow-up of 36.0 months in patients with R/R disease and 23.5 months in TN patients, the observed safety profile of single-agent zanubrutinib was consistent with both the natural history of WM and the known toxicity profile of BTK inhibitor therapy.27 Although minor bleeding was common, only 3 patients (3.9%) experienced major hemorrhagic events (hemothorax/melena, purpura, and hemorrhagic cystitis), with other contributing factors implicated in 2. This low frequency is consistent with the favorable platelet inhibition profile of zanubrutinib as compared with ibrutinib in vitro.28 Of the 4 patients with atrial fibrillation, 1 had a prior history and none required treatment discontinuation.
Infections were the most common and worrisome AE, with both zanubrutinib (treatment-related for 15 of 70 patients with events) and immunocompromise from underlying WM and prior immunochemotherapy (including fludarabine and bendamustine) contributing. These were mainly respiratory tract infections, and in most patients (67 of 70; 95.7%) they were managed without the need for treatment discontinuation. There were 4 proven fungal infections (all in patients with R/R disease); 2 were grade ≥3. Although rates were low in clinical trials, since its initial approval, there have been multiple reports of opportunistic infections caused by Pneumocystis jirovecii, Cryptococcus neoformans, and ubiquitous airborne filamentous fungi (eg, Aspergillus species) in association with ibrutinib therapy.3,24 Physicians currently consider prophylaxis in patients who are at increased risk for infections and who receive BTK inhibitor therapy.
Although commonly reported AEs observed in this study are comparable to those reported for ibrutinib, there are potential differences. Most notably, the incidence of diarrhea in patients treated with zanubrutinib (19.5%) was less than half that reported for patients with R/R WM treated with ibrutinib (42%).10 This may be related to the diminished inhibition of EGFR with zanubrutinib observed in vitro.12
Responses were observed in both patients with R/R disease and TN patients and across all MYD88/CXCR4 genotypes. For both cohorts, the rate of deep responses appears to improve with increasing treatment duration, and the longer exposure of patients with R/R disease may explain their apparently higher rate of VGPR/CR. Longer follow-up will determine whether a comparably high rate of deep responses can be achieved in TN patients. Improved VGPR rates upon longer treatment duration have previously been noted for ibrutinib in R/R WM.8 In the current study, with the limited follow-up and wide confidence intervals, achievement of deep response (VGPR/CR) was not correlated with prolonged PFS vs lesser response (PR/MR).
Recognizing the limitations of cross-study comparisons and small sample sizes, the proportion of patients with R/R disease who achieved a deep response in the current study (51%) compares favorably with that reported for patients in the ibrutinib pivotal trial (27%) despite the significantly longer treatment duration in the latter study (median, 46.6 months).8 TN patients in this study (including 3 with MYD88WT disease and 1 with an unknown genotype) also demonstrated a high rate of early deep responses (33.3%).9 The rate of CXCR4 mutations in this study was also somewhat lower than that reported in ibrutinib trials; however, the lack of B-cell selection in bone marrow aspirates may have contributed to an under-recognition in this study.
Across both cohorts, MRRs were comparably high in patients with disease harboring the MYD88L265P mutation with (90.9%) or without (87.2%) an accompanying CXCR4WHIM mutation. MRRs reported from the ibrutinib pivotal study were comparable to those in the current study for patients with MYD88L265P/CXCR4WT genotype WM (97%) but possibly lower for those with MYD88L265P/CXCR4WHIM disease (67% and 71% in R/R and TN cohorts, respectively).8,29 Although the MRRs did not differ between the aforementioned genotypes in the current study, there was a clear difference in the rate of deep responses, favoring patients with MYD88L265P/CXCR4WT genotype disease (Figure 1). Among patients with MYD88WT disease, 2 of 8 patients (25.0%) achieved a deep response (including the only patient across both cohorts achieving a CR) and 5 patients (62.5%) achieved a major response. Although these data indicate meaningful clinical benefit for patients across all MYD88/CXCR4 genotypes (including MYD88WT), small sample sizes warrant caution in their interpretation.
In summary, these results showed that long-term treatment with zanubrutinib resulted in deep and durable responses in the majority of patients with WM and was well tolerated. Deep responses were seen in patients with both TN and R/R disease and in all molecular subtypes, including MYD88WT. As a potent and selective BTK inhibitor, zanubrutinib offers the potential for improved efficacy, safety, and tolerability over existing treatment options. To address this question, a randomized phase 3 study comparing the safety and efficacy of zanubrutinib with ibrutinib in patients with WM is underway (NCT03053440).
Individual participant data will not be shared prior to regulatory approval of zanubrutinib for the treatment of Waldenström macroglobulinemia. Requests for copies of the protocol and statistical analysis plan will be considered (judith.trotman@health.nsw.gov.au).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in the study, their supporters, and the investigators and clinical research staff from the study centers.
This study was supported by research funding from BeiGene (Beijing) Co., Ltd. (Beijing, China). Medical writing and editorial assistance were funded by BeiGene and provided by Gordon Bray and Bio Connections (Chicago, IL).
Authorship
Contribution: Together with BeiGene authors (W.N., J.D.H., J.H., S.K.A., E.H., Z.T., and Y.Y.), C.S.T., J.T., J.F.S., S.O., and A.W.R. were responsible for study design; J.T., S.O., D.G., D.S., P.M., J.M., A.W.R., J.F.S., and C.S.T. contributed to data interpretation and analysis; all investigators (J.T., S.O., D.G., D.S., P.M., G.C., J.M., A.T., A.W.R., J.F.S., and C.S.T.) and their respective research teams reviewed patient records and contributed to data collection; BeiGene authors J.D.H., E.H., and Z.T. confirmed the data accuracy and compiled the data for summation and analysis; J.D.H., E.H., Z.T., S.K.A., and Y.Y. performed data analysis and interpretation; all authors contributed to the manuscript preparation; BeiGene was involved in the study design, compilation of data, and statistical analysis; the corresponding author, J.T., had the final responsibility to submit the manuscript for publication; all authors had full access to all of the data; and all authors carefully reviewed the manuscript and approved the final version.
Conflict-of-interest disclosure: J.T. receives research funding from BeiGene, Janssen, Celgene, Pharmacyclics, and Roche. S.O. consults for AbbVie, Janssen, Gilead, Roche, Mundipharma, Merck, BMS, and Celgene; receives research funding from AbbVie, BeiGene, Janssen, Gilead, Roche, Celgene, and Epizyme; and receives honoraria from AbbVie, Janssen, Gilead, Roche, Mundipharma, Merck, BMS, and Celgene. D.G. is an employee of the University of Sydney; consults for Novartis, Gilead, AbbVie, and Merck; receives research funding from Haemalogix P/L; and serves on advisory committees for Haemalogix P/L. D.S. receives research funding from Amgen, BeiGene, AbbVie, Roche, Celgene, MSD, Acerta, Pharmacyclics, Sanofi, and Glaxo-Smith-Kline and receives honoraria from Janssen, Roche, and AbbVie. P.M. receives honoraria from Celgene, Roche, and AbbVie and serves on advisory committees for AbbVie, Roche, Novartis, Janssen, Astellas, and Celgene. G.C. receives travel, accommodations, and expenses from Amgen, Glycomimetics, and AbbVie. J.M. consults for Pharmacyclics, Bayer, Gilead/Kite Pharma, Pfizer, Janssen, Juno/Celgene, BMS, Kyowa, Alexion, BeiGene, and Seattle Genetics; receives research funding from Kite Pharma, Celgene, Portola, Incyte, Genentech, Pharmacyclics, Seattle Genetics, and Janssen; receives honoraria from Kyowa and Seattle Genetics; and participates in a speakers bureau for BeiGene, Kite Pharma, Gilead, Fosunkite, Kyowa, Bayer, Pharmacyclics, and AstraZeneca. A.T. receives honoraria from BeiGene, participates in a speakers bureau for Janssen Cilag SpA, and serves on advisory committees for Janssen Cilag SpA, Sunesis, and Astra Zeneca. A.W.R. receives research funding from AbbVie and Janssen and receives royalties from Walter and Eliza Hall Institute. J.F.S. consults for Roche; receives research funding from AbbVie, Celgene, Janssen, and Roche; receives honoraria from AbbVie, Acerta, Celgene, Gilead, Janssen, Roche, and Takeda; participates in a speakers bureau for AbbVie and Roche; and serves on advisory committees for AbbVie, Acerta, Celgene, Gilead, Janssen, Roche, and Takeda. S.K.A., E.H., Z.T., Y.Y., W.N., J.D.H., and J.H. are employees of and have equity ownership in BeiGene. C.S.T. receives research funding from Janssen and AbbVie and receives honoraria from Janssen, AbbVie, BeiGene, Novartis, and Roche.
Correspondence: Judith Trotman, Haematology Department, Concord Repatriation General Hospital, University of Sydney, Hospital Rd, Concord NSW 2139, Australia; e-mail: judith.trotman@health.nsw.gov.au.