In this issue of Blood, Subramaniam et al reveals that UM171 mechanistically facilitates expansion of human umbilical cord blood (UCB) hematopoietic stem cells (HSCs) by targeting the proteo-degradation of lysine-specific histone demethylase 1A (LSD1) and also targeting the RCOR1 subunit of CoREST.1
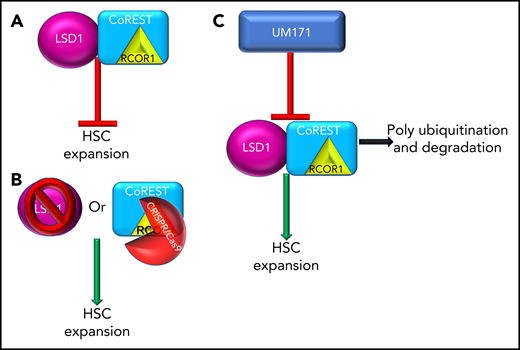
LSD1 and CoREST restrict ex vivo propagation of human HSCs and are targets of UM171. (A) LSD and/or the CoREST complex inhibit ex vivo expansion of HSCs. (B) Inhibition of LSD1 (pharmacological or CRISPR/Cas9) or inhibition of the CoREST core member, RCOR1 (via CRISPR/Cas9), resulted in ex vivo expansion of HSCs. (C) UM171 treatment results in polyubiquitination and degradation of LSD1 and CoREST leading to ex vivo expansion of HSCs.
LSD1 and CoREST restrict ex vivo propagation of human HSCs and are targets of UM171. (A) LSD and/or the CoREST complex inhibit ex vivo expansion of HSCs. (B) Inhibition of LSD1 (pharmacological or CRISPR/Cas9) or inhibition of the CoREST core member, RCOR1 (via CRISPR/Cas9), resulted in ex vivo expansion of HSCs. (C) UM171 treatment results in polyubiquitination and degradation of LSD1 and CoREST leading to ex vivo expansion of HSCs.
Throughout life, HSCs provide multipotent differentiation leading to production of functional immune cells and self-renewal of the blood cell pool. Allogeneic transplantation of HSCs has been used since the 1960s to provide long-term reconstitution of the marrow for recipients. Multiple sources of HSCs are used, including bone marrow aspirates, mobilized peripheral stem cells, and UCB. Each stem cell source has different limitations, including prolonged time to neutrophil/platelet recovery and limited number of hematopoietic stem and progenitor cells (HSPCs) for transplant, especially in the case of UCB. In addition, the number of HSCs/HSPCs (CD34+) transplanted has been directly correlated with engraftment and, consequently, long-term patient survival. Due to these clinical difficulties, the field of HSC expansion was born. The ability to ex vivo expand these cells for clinical use is important, and multiple methods have been extensively investigated. However, the mechanisms by which ex vivo expansion occurs are often unclear or completely unknown.
Regulation of HSC self-renewal and differentiation is tightly regulated under a complex interplay of both intrinsic and extrinsic factors. As UCB units are limited, strategies to ex vivo expand progenitors available for initial engraftment, short-term multilineage reconstitution, platelet/neutrophil recovery, and enhancing the number of long-term HSCs to establish long-term engraftment are needed. Many different strategies in ex vivo culture systems have been shown to promote HSC expansion. These include small molecules, such as UM171 and SR1, cytokines/growth factors (Flt3, SCF, TPO), extracellular matrices/cell cocultures, and alterations to transgene overexpression, such as HOXB4.2 With small-molecule inhibitors, many different drugs targeting p38, histone deactylase signaling, Notch, prostaglandin E2, aryl hydrocarbon receptor, and pyrimidoindole derivatives, etc, have been shown to promote expansion. However, in many cases, they only expand human, not murine, HSCs. There is often little mechanistic insight into their function or to the targets that lead to modified self-renewal or differentiation blocks.
The pyrimidoindole derivative UM171 was initially identified as a potent ex vivo expander of HSCs that did not suppress the aryl hydrocarbon receptor.3,4 Subsequent studies have shown that UM171 expands distinct myeloid/lymphoid progenitors, enhances derivation of HSPCs from human pluripotent stem cells, and increases lentiviral gene transfer and recovery of HSCs.5-7 Clinically, feasibility of engraftment of single-unit CB expanded with UM171 was demonstrated, thus overcoming the shortcomings of UCB as a stem cell source while maintaining the benefit of a low incidence of graft-versus-host disease.8 Despite the numerous scientific studies and evident clinical relevance of UM171, the specific mechanism(s) by which it functions to ex vivo expand UCB and maintain HSCs self-renewal and function remained uncertain. UM171 influences the balance between pro- and anti-inflammatory regulation via NF-κB activation and protein C receptor–dependent reactive oxygen species (ROS) detoxification, which could potentially explain some of its effects.9
The elegantly direct study by Subramaniam et al investigated the interactive mechanisms of UM171 enhancement of HSC expansion. The authors implemented both inhibitor and CRISPR/Cas9 techniques and focused on LSD1 and CoREST as the primary targets of UM171. LSD1, when integrated in the CoREST/Rcor1 complex, regulates gene expression via elimination of mono-/dimethyl groups on H3 lysine K4 and K9 residues. The authors chose to explore LSD1 because LSD1 has been shown to expand murine HSPC in vivo, and LSD1 conditional knockout results in expansion of HSPC in the marrow and restriction of hematopoietic differentiation.1,10 In this study by Subramaniam et al, inhibition of LSD1 via (1) chemical inhibition (2-PCPA), (2) CRISPR/Cas9 knockout of LSD1, or the CoREST core member RCOR1, resulted in expansion of frequency and number of CD34+EPCR+ cells in both UCB and adult human bone marrow, with strong enhancement of HSC-associated genes (UCB). Transplantation analysis of both LSD1 inhibition and UM171 pretreated/expanded UCB resulted in enhanced chimerism and numbers of SCID-repopulating cells compared with dimethyl sulfoxide control. Most importantly, they detected that UM171 induced abrupt and proficient proteasomal-mediated degradation of LSD1 and the CoREST complex, especially RCOR1.
Altogether, this study is the first-of-its-kind, in-depth investigation of the mechanistic regulation, and balance, of HSC/HSPC expansion, propagation, fate, and engraftment of HSC/HSPC via UM171 (see figure). Furthermore, these data provide a foundation for future studies of mechanistic targets to increase expansion and clinical manipulation of normal and malignant stem cells. The potential long-term, clinical, and vast cell-type implications of these responses will be important avenues for future investigations.
Conflict-of-interest disclosure: The author declares no competing financial interests.