Abstract
Pregnancy and postpartum are high-risk periods for different forms of thrombotic microangiopathy (TMA). However, the management of pregnancy-associated TMA remains ill defined. This report, by an international multidisciplinary working group of obstetricians, nephrologists, hematologists, intensivists, neonatologists, and complement biologists, summarizes the current knowledge of these potentially severe disorders and proposes a practical clinical approach to diagnose and manage an episode of pregnancy-associated TMA. This approach takes into account the timing of TMA in pregnancy or postpartum, coexisting symptoms, first-line laboratory workup, and probability-based assessment of possible causes of pregnancy-associated TMA. Its aims are: to rule thrombotic thrombocytopenic purpura (TTP) in or out, with urgency, using ADAMTS13 activity testing; to consider alternative disorders with features of TMA (preeclampsia/eclampsia; hemolysis elevated liver enzymes low platelets syndrome; antiphospholipid syndrome); or, ultimately, to diagnose complement-mediated atypical hemolytic uremic syndrome (aHUS; a diagnosis of exclusion). Although they are rare, diagnosing TTP and aHUS associated with pregnancy, and postpartum, is paramount as both require urgent specific treatment.
Introduction
Thrombotic microangiopathy (TMA) is a potentially severe disorder defined by a pattern of endothelial cell injury.1 Pregnancy and postpartum have long been recognized as high-risk periods for different forms of TMA.2 In the last 2 decades, several breakthroughs in the understanding and thus the treatment of various forms of TMA have improved the outcome, including forms of TMA associated with pregnancy and the postpartum period.
This has prompted the creation of an international multidisciplinary working group of experts (obstetricians, nephrologists, hematologists, intensivists, neonatologists and complement biologists) in the field of TMA occurring in the pregnancy period. The present report of this group aims to summarize current knowledge of these severe disorders and to provide practical guidance for clinicians.
The spectrum of pregnancy-associated TMA
TMA refers to a pattern of endothelial cell injury of variable severity that preferentially affects the kidneys, brain, and the heart1,3 (Table 1). However, diagnosis is most frequently based on clinical and biological data constituting a classic triad of peripheral thrombocytopenia, mechanical hemolytic anemia, and organ dysfunction, particularly in the central nervous system (altered consciousness, seizures), the kidneys (acute kidney injury [AKI]), and the heart (raised serum troponin level, ischemia, sudden death).
Definition of TMA and of the main conditions associated with features of TMA during pregnancy and postpartum
| Main conditions associated with features of TMA during pregnancy and postpartum . |
|---|
| Thrombotic microangiopathy (TMA) |
| TMA is defined by a pathological pattern1,3 ; endothelial cell swelling and detachment from the basement membrane, thrombi in the microcirculation and, in the kidney, “double contour” aspects of the glomerular basement membrane and a dissolution or attenuation of the mesangial matrix (mesangiolysis); it is however usually diagnosed based on a clinicopathological triad: |
| (a) peripheral thrombocytopenia (platelet count <100 × 109/L) |
| (b) mechanical hemolytic anemia (hemoglobin <10 g/dL, LDH >upper limit of normal, undetectable haptoglobin, schistocytes on blood smear) |
| (c) organ injury |
| Thrombotic thrombocytopenic purpura (TTP) |
| TMA with mainly hematological, neurological, and potentially cardiac involvement |
| Usually associated to a complete hereditary or immune deficiency in ADAMTS13 plasma activity |
| Hemolytic uremic syndrome (HUS) |
| TMA with mainly renal involvement and potentially neurological and cardiac involvement |
| May be linked to various types of endothelial cell injury1,107 |
| Atypical HUS is caused by a dysregulation of the complement alternative pathway1,107 |
| HUS can include extrarenal manifestations and TTP may be associated with significant renal disease, it may prove difficult to distinguish the 2 entities on clinical grounds alone |
| Preeclampsia/eclampsia (PE/E) and hemolysis, elevated liver enzymes and low platelet count (HELLP) syndrome |
| PE is defined as gestational hypertension (systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg) accompanied by ≥1 of the following new-onset conditions at ≥20 weeks’ gestation4 : |
| Proteinuria |
| Acute kidney injury (serum creatinine ≥90 μmol/L) |
| Alanine or aspartate aminotransferase >40 IU/L ± right upper quadrant or epigastric abdominal pain |
| Eclampsia, in addition to the criteria defining PE, is characterized by altered mental status, blindness, stroke, clonus, severe headaches, and persistent visual scotomata |
| Platelet count <100 g/L, disseminated intravascular coagulation, hemolysis |
| Fetal growth restriction, abnormal umbilical artery Doppler wave form analysis, or stillbirth |
| HELLP syndrome is considered part of PE/E4,5 (the most severe part of the spectrum of PE/E) |
| PE/E and HELLP syndrome are associated with an imbalance between angiogenic (placental growth factor) and antiangiogenic (soluble Flt1) factors |
| To date, PE/E and HELLP syndrome have not been linked to acquired or hereditary severe (<20%) deficiency in ADAMTS13 activity nor to a hereditary complement dysregulation |
| Catastrophic antiphospholipid syndrome (CAPS) |
| CAPS is defined by the occurrence of fulminant multiorgan damage (brain, kidney, lung, skin, etc) resulting from extensive small vessel thrombosis in the setting of persistent antiphospholipid antibodies (lupus anticoagulant, anticardiolipin, and anti-b2GPI antibodies)7 ; it is usually associated with thrombocytopenia and mechanical hemolytic anemia |
| Main conditions associated with features of TMA during pregnancy and postpartum . |
|---|
| Thrombotic microangiopathy (TMA) |
| TMA is defined by a pathological pattern1,3 ; endothelial cell swelling and detachment from the basement membrane, thrombi in the microcirculation and, in the kidney, “double contour” aspects of the glomerular basement membrane and a dissolution or attenuation of the mesangial matrix (mesangiolysis); it is however usually diagnosed based on a clinicopathological triad: |
| (a) peripheral thrombocytopenia (platelet count <100 × 109/L) |
| (b) mechanical hemolytic anemia (hemoglobin <10 g/dL, LDH >upper limit of normal, undetectable haptoglobin, schistocytes on blood smear) |
| (c) organ injury |
| Thrombotic thrombocytopenic purpura (TTP) |
| TMA with mainly hematological, neurological, and potentially cardiac involvement |
| Usually associated to a complete hereditary or immune deficiency in ADAMTS13 plasma activity |
| Hemolytic uremic syndrome (HUS) |
| TMA with mainly renal involvement and potentially neurological and cardiac involvement |
| May be linked to various types of endothelial cell injury1,107 |
| Atypical HUS is caused by a dysregulation of the complement alternative pathway1,107 |
| HUS can include extrarenal manifestations and TTP may be associated with significant renal disease, it may prove difficult to distinguish the 2 entities on clinical grounds alone |
| Preeclampsia/eclampsia (PE/E) and hemolysis, elevated liver enzymes and low platelet count (HELLP) syndrome |
| PE is defined as gestational hypertension (systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg) accompanied by ≥1 of the following new-onset conditions at ≥20 weeks’ gestation4 : |
| Proteinuria |
| Acute kidney injury (serum creatinine ≥90 μmol/L) |
| Alanine or aspartate aminotransferase >40 IU/L ± right upper quadrant or epigastric abdominal pain |
| Eclampsia, in addition to the criteria defining PE, is characterized by altered mental status, blindness, stroke, clonus, severe headaches, and persistent visual scotomata |
| Platelet count <100 g/L, disseminated intravascular coagulation, hemolysis |
| Fetal growth restriction, abnormal umbilical artery Doppler wave form analysis, or stillbirth |
| HELLP syndrome is considered part of PE/E4,5 (the most severe part of the spectrum of PE/E) |
| PE/E and HELLP syndrome are associated with an imbalance between angiogenic (placental growth factor) and antiangiogenic (soluble Flt1) factors |
| To date, PE/E and HELLP syndrome have not been linked to acquired or hereditary severe (<20%) deficiency in ADAMTS13 activity nor to a hereditary complement dysregulation |
| Catastrophic antiphospholipid syndrome (CAPS) |
| CAPS is defined by the occurrence of fulminant multiorgan damage (brain, kidney, lung, skin, etc) resulting from extensive small vessel thrombosis in the setting of persistent antiphospholipid antibodies (lupus anticoagulant, anticardiolipin, and anti-b2GPI antibodies)7 ; it is usually associated with thrombocytopenia and mechanical hemolytic anemia |
GPI, glycoprotein I.
The commonest causes of mechanical hemolytic anemia and thrombocytopenia with organ involvement in pregnancy are preeclampsia (PE)/eclampsia and hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome (as recently defined4 ; Table 1), which are part of the same syndrome with different presentation and severity.5 Acute fatty liver disease of pregnancy may also have some features of TMA.6 More rarely, pregnancy-associated TMA is related to hemolytic uremic syndrome (HUS; TMA with predominant renal involvement) or thrombotic thrombocytopenic purpura (TTP; TMA with predominant hematological and neurological involvement) (Table 1). The characteristic triad can also be found in the setting of severe autoimmune diseases, mainly systemic lupus erythematosus (SLE) and the catastrophic antiphospholipid syndrome (CAPS).7 In the latter case, antiphospholipid syndrome (APS) may be known before pregnancy.8
The diagnosis of pregnancy-associated TMA
TMA in pregnancy is diagnosed based on the presence of a platelet count <100 × 109/L, a hemoglobin level <10 g/dL, a lactate dehydrogenase (LDH) serum level >1.5 upper limit of normal, undetectable serum haptoglobin, negative direct erythrocyte antiglobulin test and (1) the presence of schistocytes on blood smear or (2) TMA features in kidney (or another organ) biopsy.
The platelet count decreases during normal pregnancy and ∼10% of patients with uncomplicated pregnancies have a platelet count <150 × 109/L at the time of delivery.9,10 Thus, a platelet count threshold of <100 × 109/L is appropriate for the diagnosis of pregnancy-associated TMA. AKI is frequently encountered in most types of pregnancy-associated TMA, except TTP. There is no universally accepted definition of AKI during pregnancy. The various available definitions refer to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines11 : a doubling or >26 μmol/L increase in serum creatinine. The definition used in this report is based on a serum creatinine >90 μmol/L and/or a >25% increase compared with baseline values.
A mechanistic approach to pregnancy/postpartum-associated TMA
The mechanisms underlying the 2 main forms of TMA, TTP and HUS, have been dissected over the past 2 decades (presented in details in Figure 1).
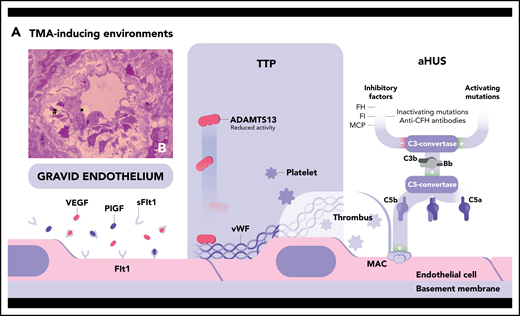
Pathogenic mechanisms of 2 main forms of TMA. (A) TTP is linked to a severe deficiency in ADAMTS13, a metalloproteinase produced by the liver that specifically cleaves the ultralarge multimers of VWF (ULVWF), the most hemostatically active species of VWF. ADAMTS13 deficiency may be acquired (inhibitory autoantibodies directed against ADAMTS13 in immune TTP) or hereditary (recessively inherited biallelic mutations of the encoding gene in hereditary TTP) (Upshaw-Schulman syndrome) (∼3% of cases13 ). Consequently, ADAMTS13 deficiency leads to the accumulation in the circulation of platelet-hyperadhesive ULVWF multimers with ensuing spontaneous formation of microthrombi within the microcirculation, the fragmentation of red blood cells projected against thrombi, and organ ischemic damage. HUS may be triggered by different mechanisms of endothelial cell injury leading to a common activated prothrombotic phenotype of these cells.1,107 The most frequent forms of HUS are Shiga toxin–producing E coli–associated HUS (STEC-HUS; resulting from Shiga-like toxin–induced endothelial damage) and secondary HUS associated with malignancy, drugs, autoimmune disease, or infection.108 Complement-mediated atypical HUS (aHUS) is linked to a dysregulation of the complement alternative pathway. Alternative C3 convertase, a key enzyme of the alternative pathway composed mainly of C3b and Bb, is in state of continuous low-grade activation. Three main inhibitors tightly control this enzyme: factor H (FH), a circulating protein that attaches to normal endothelial cells, and the membrane-bound membrane-cofactor protein (MCP), which both bind C3b; and factor I (FI), which cleaves C3b. Hyperactivation of the alternative C3 convertase may result from inactivating mutation in FH-, FI-, and MCP-encoding genes or activating mutations in FB- and C3-encoding genes. Uncontrolled activation of the C3 convertase leads to the generation of the C5 convertase and the cleavage of C5 into C5a and C5b. This initiates the formation at the surface of the endothelial cell of the membrane-attack complex (MAC), a cytotoxic multiproteic structure. Complement-induced endothelial cell damage and activation promote thrombi formation and the TMA process. During pregnancy, the occurrence of TMA may be facilitated by the peculiar phenotype of “gravid endothelium,” resulting from the antiangiogenic state characteristic of pregnancy: the relative imbalance between the angiogenic vascular endothelial growth factor (VEGF), the placental growth factor (PlGF), and the antiangiogenic soluble fms-like tyrosine kinase-1 (sFlt1). TMA is the consequence of a phenotype of activated endothelial cell that leads to endothelial cell swelling (*) and detachment (#) from the basement membrane, as shown by light microscopy in the semi-thin kidney biopsy section in panel B (toluidine blue stain; original magnification ×100), and ultimately to thrombosis.
Pathogenic mechanisms of 2 main forms of TMA. (A) TTP is linked to a severe deficiency in ADAMTS13, a metalloproteinase produced by the liver that specifically cleaves the ultralarge multimers of VWF (ULVWF), the most hemostatically active species of VWF. ADAMTS13 deficiency may be acquired (inhibitory autoantibodies directed against ADAMTS13 in immune TTP) or hereditary (recessively inherited biallelic mutations of the encoding gene in hereditary TTP) (Upshaw-Schulman syndrome) (∼3% of cases13 ). Consequently, ADAMTS13 deficiency leads to the accumulation in the circulation of platelet-hyperadhesive ULVWF multimers with ensuing spontaneous formation of microthrombi within the microcirculation, the fragmentation of red blood cells projected against thrombi, and organ ischemic damage. HUS may be triggered by different mechanisms of endothelial cell injury leading to a common activated prothrombotic phenotype of these cells.1,107 The most frequent forms of HUS are Shiga toxin–producing E coli–associated HUS (STEC-HUS; resulting from Shiga-like toxin–induced endothelial damage) and secondary HUS associated with malignancy, drugs, autoimmune disease, or infection.108 Complement-mediated atypical HUS (aHUS) is linked to a dysregulation of the complement alternative pathway. Alternative C3 convertase, a key enzyme of the alternative pathway composed mainly of C3b and Bb, is in state of continuous low-grade activation. Three main inhibitors tightly control this enzyme: factor H (FH), a circulating protein that attaches to normal endothelial cells, and the membrane-bound membrane-cofactor protein (MCP), which both bind C3b; and factor I (FI), which cleaves C3b. Hyperactivation of the alternative C3 convertase may result from inactivating mutation in FH-, FI-, and MCP-encoding genes or activating mutations in FB- and C3-encoding genes. Uncontrolled activation of the C3 convertase leads to the generation of the C5 convertase and the cleavage of C5 into C5a and C5b. This initiates the formation at the surface of the endothelial cell of the membrane-attack complex (MAC), a cytotoxic multiproteic structure. Complement-induced endothelial cell damage and activation promote thrombi formation and the TMA process. During pregnancy, the occurrence of TMA may be facilitated by the peculiar phenotype of “gravid endothelium,” resulting from the antiangiogenic state characteristic of pregnancy: the relative imbalance between the angiogenic vascular endothelial growth factor (VEGF), the placental growth factor (PlGF), and the antiangiogenic soluble fms-like tyrosine kinase-1 (sFlt1). TMA is the consequence of a phenotype of activated endothelial cell that leads to endothelial cell swelling (*) and detachment (#) from the basement membrane, as shown by light microscopy in the semi-thin kidney biopsy section in panel B (toluidine blue stain; original magnification ×100), and ultimately to thrombosis.
Pregnancy-associated TTP usually results from a severe deficiency in ADAMTS13, the specific metalloproteinase that cleaves ultralarge (UL) multimers of von Willebrand factor (VWF). It accounts for 10% to 30% of all adult cases of TTP12-16 (17% of all TTP cases occur in women of childbearing age14 ). Severe ADAMTS13 deficiency (enzyme level <20%) is documented in 1 in 17 000 to 1 in 200 000 pregnancies, making TTP a rare complication of pregnancy.14,17 In addition, pregnancy is a recognized trigger of adult-onset TTP in women with genetic ADAMTS13 deficiency. In a recent study, the proportion of hereditary TTP patients (24%) among pregnancy-associated TTP cases was much higher than that in adult-onset TTP in general (<5%).14 Most cases of pregnancy-associated hereditary TTP were linked to a cluster of ADAMTS13 variants.14
In normal pregnancy, ADAMTS13 activity progressively and steadily decreases by ∼50% from the second trimester until delivery18 (probably because of an increased release of VWF). Decreased ADAMTS13 activity (∼20% to 40%) has also been reported in patients with PE/eclampsia, HELLP syndrome or pregnancy-associated HUS,19,20 but generally remains detectable. Thus, only pregnancy-associated TTP is defined by a severe deficiency (<20%) in ADAMTS13 activity, which tends to occur most frequently during the second and third trimesters21 (Case 1).
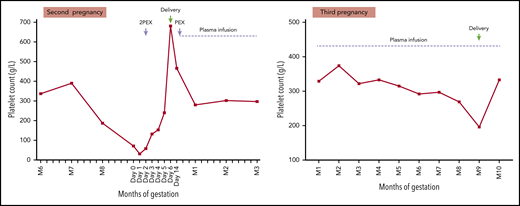
A 38-year-old woman had a first pregnancy that ended at 24 weeks of gestation in intrauterine fetal death associated with severe intrauterine growth retardation and ischemic changes in the placenta. No blood counts were undertaken at that time. During her second pregnancy, she received empirical low-dose aspirin and low-molecular-weight heparin prophylaxis. A full blood count was normal at the start of pregnancy. She was referred at 34 weeks of gestation because of an increasing frequency of migraines with visual disturbance, severe lethargy, and depression. Laboratory tests showed lowest platelet count (71 × 109/L), with increased LDH, schistocytes on blood film, and normal serum creatinine. ADAMTS13 was undetectable (<5 IU/dL) but no anti-ADAMTS13 antibody was detected. Hereditary TTP was diagnosed. She received 2 plasma exchanges and had delivery by caesarean section without any platelet transfusion. A further plasma exchange was performed after delivery and subsequently she received plasma infusions. Placental histology showed no ischemic changes. During her third pregnancy, she received low-dose aspirin and low-molecular-weight heparin prophylaxis and plasma infusions throughout pregnancy, with an increase in infusion volume from the second trimester and frequency (twice per week) from the third trimester. She delivered a healthy infant by caesarean section at 35 weeks of gestation.
Pregnancy-associated HUS is a similarly rare disorder.22 In the largest published series, it represented 16% of all atypical HUS (aHUS) cases occurring in women aged 18 to 45 years,23 when the estimated incidence of aHUS in the general population is ∼0.5 in 106 people.1 Pregnancy-associated HUS was long considered as part of the spectrum of secondary HUS. Nevertheless, several studies21,23,24 have established that pregnancy-associated HUS and complement-mediated aHUS have the same severe presentation (41% to 71% of patients requiring dialysis) and poor renal outcomes in the absence of specific treatment (end-stage renal disease in 53% of cases) and a similar frequency of complement gene variants (41% to 56% of cases)23,24 . Thus, it is currently accepted that pregnancy-associated HUS belongs to the spectrum of complement-mediated aHUS.
Pregnancy-associated HUS is the only form of TMA to occur most frequently (three-fourths of cases) in the postpartum period (up to 3 months after delivery), and TMA starting in the postpartum of an uneventful pregnancy is very suggestive of complement-mediated aHUS21,24 (Case 2). However, pregnancy-associated HUS may occur during any trimester.
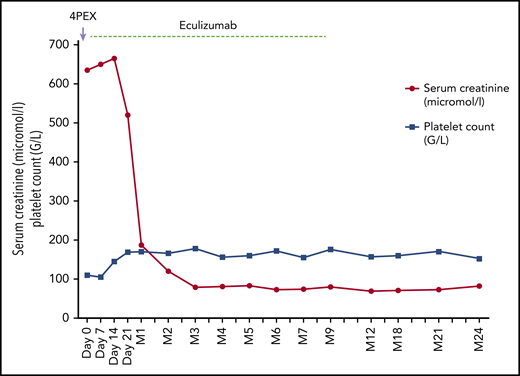
A 34-year-old woman delivered a healthy male infant at 38 weeks of gestation of her second uneventful pregnancy. She was discharged to home 5 days later. Three weeks postpartum, she was referred for asthenia. Her blood pressure at admission was 158/92 mm Hg. Physical examination was unremarkable. Laboratory tests showed: serum creatinine, 635 μmol/L; platelet count, 110 × 109/L; hemoglobin, 10 g/dL; LDH level, 2.5 upper limit of normal; and undetectable haptoglobin. Proteinuria was 2 g/L. Schistocytes were detected on blood smear. The patient was started on plasma exchanges with no effect on serum creatinine or platelet count. Further workup showed normal C3, C4, and total complement hemolytic 50%. ADAMTS13 activity was decreased at 39% but detectable. Tests for antinuclear, anti-native DNA, anti-cardiolipin, and anti-b2GPI antibodies and for lupus anticoagulant were negative. The diagnosis of aHUS was made by exclusion. Five days after admission, plasma exchanges were discontinued and anti-C5 treatment (eculizumab) was started. Platelet count rapidly normalized and renal function gradually recovered. Three months later, genetic tests disclosed a likely pathogenic variant in the complement factor H gene (c.773 C>T;p.Pro258Leu). Anti-C5 treatment was discontinued after 9 months of treatment. No recurrence of aHUS occurred. The patient had 2 subsequent uneventful pregnancies.
Even though the mechanisms of pregnancy-associated TTP and HUS have been clarified, pregnancy-associated TMA still raises challenging diagnostic and therapeutic issues. On the one hand, PE/eclampsia and HELLP syndrome, which are far more frequent than TTP and aHUS,22 may present with clinical and laboratory features of TMA that spontaneously resolve after delivery in the vast majority of cases (Case 3). On the other hand, patients with pregnancy-associated aHUS (and to a lesser extent those with TTP) frequently present with severe hypertension, proteinuria, and AKI as first symptoms, and may mimic PE/eclampsia or HELLP syndrome. Moreover, pregnancy in patients with well-characterized aHUS25 or TTP26,27 may be complicated by PE/eclampsia or HELLP syndrome.
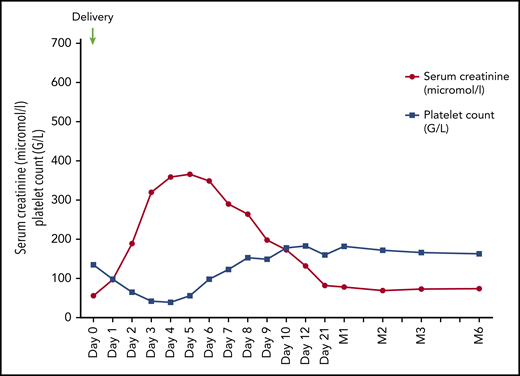
A 27-year-old woman presented with hypertension (168/97 mm Hg) and proteinuria (1.7 g/L) at 33 weeks of gestation of her first pregnancy. She complained of persistent headaches. The following day, proteinuria worsened (4.2 g/L); abnormal fetal cardiac rhythm was detected. Serum sFlt1/PlGF ratio was 48. The patient underwent a caesarean section and gave birth to a female infant (1760 g). Delivery was complicated by severe bleeding requiring red blood cells (6 concentrates) and platelet transfusion (2 concentrates), the administration of tranexamic acid (total dose, 2 g), and uterine revision. She rapidly developed AKI (peak serum creatinine, 366 μmol/L), severe oliguria, profound thrombocytopenia (nadir, 39 g/L) with elevated LDH level (3.7 times the upper limit of normal), undetectable haptoglobin, and presence of schistocytes on blood smear. ADAMTS13 activity was 29%. As the most probable diagnosis was preeclampsia and/or postpartum hemorrhage–associated TMA, no specific treatment was implemented. The patient was closely monitored in the ICU and received IV calcium inhibitor to normalize her blood pressure. Renal magnetic resonance imaging excluded renal cortical necrosis. Serum creatinine and platelet count gradually improved starting day 6 and the patient was discharged on day 12 with mild residual renal function impairment, normal platelet count, persistent anemia (hemoglobin, 9.8 g/dL), and decreased haptoglobin (0.3 g/L; normal 0.5-2.2 g/L). Nine days later, all of her renal and hematological parameters had normalized.
Finally, TMA may be associated with exacerbation of autoimmune systemic disorders, including CAPS during pregnancy and postpartum. The identification of this potential cause of TMA is crucial for the implementation of specific treatments (high-dose steroids, anticoagulation, plasma exchange, and cyclophosphamide28 ).
Practical clinical management of pregnancy-associated TMA
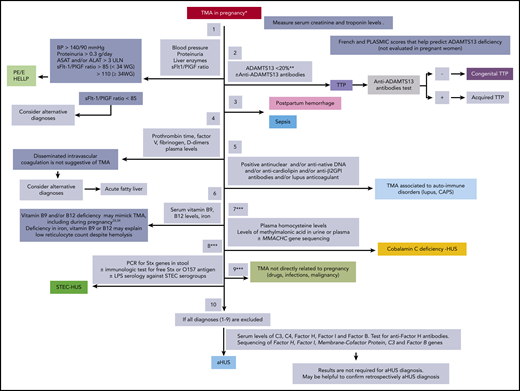
Practical clinical management of a patient with pregnancy-associated TMA should take into account the timing of TMA in pregnancy or postpartum (supplemental Figure 1, available on the Blood Web site), coexistent symptoms, first-line laboratory workup (Figure 21,29-34 ; supplemental Table 1), probability-based assessment of causes of pregnancy-associated TMA, and general characteristics of this disorder (Table 214,23,25,35-42 ): all of these parameters guide treatment (Figure 3). This management should ideally take place in a tertiary referral center.
Algorithm for the initial biological workup in women with TMA occurring during pregnancy and the postpartum. Different causes of pregnancy-associated TMA are to be ruled in or out (from top to bottom) before the diagnosis of atypical HUS is made. The most likely diagnoses are shown in full colored rectangles. *TMA in pregnancy is diagnosed based on the presence of a platelet count <100 × 109/L, a hemoglobin level <10 g/dL, LDH >1.5 upper limit of normal, undetectable serum haptoglobin, negative direct erythrocyte antiglobulin test, and (1) the presence of schizocytes on blood smear or (2) TMA features in kidney biopsy. **Cutoff proposed by the working group. May vary according to the diagnostic assay used in each expert laboratory. ***These steps are optional and are performed on a case-by-case basis. ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase; BP, blood pressure; GPI, glycoprotein I; HELLP, hemolysis elevated liver enzymes and low platelet count; LPS, lipopolysaccharide; MMACHC, methylmalonic aciduria and homocystinuria type C protein; PCR, polymerase chain reaction; PlGF, placental growth factor; PE/E, preeclampsia/eclampsia; sFlt1, soluble fms-like tyrosine kinase-1; Stx, Shiga toxin; ULN, upper limit of normal; WG, weeks of gestation.
Algorithm for the initial biological workup in women with TMA occurring during pregnancy and the postpartum. Different causes of pregnancy-associated TMA are to be ruled in or out (from top to bottom) before the diagnosis of atypical HUS is made. The most likely diagnoses are shown in full colored rectangles. *TMA in pregnancy is diagnosed based on the presence of a platelet count <100 × 109/L, a hemoglobin level <10 g/dL, LDH >1.5 upper limit of normal, undetectable serum haptoglobin, negative direct erythrocyte antiglobulin test, and (1) the presence of schizocytes on blood smear or (2) TMA features in kidney biopsy. **Cutoff proposed by the working group. May vary according to the diagnostic assay used in each expert laboratory. ***These steps are optional and are performed on a case-by-case basis. ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase; BP, blood pressure; GPI, glycoprotein I; HELLP, hemolysis elevated liver enzymes and low platelet count; LPS, lipopolysaccharide; MMACHC, methylmalonic aciduria and homocystinuria type C protein; PCR, polymerase chain reaction; PlGF, placental growth factor; PE/E, preeclampsia/eclampsia; sFlt1, soluble fms-like tyrosine kinase-1; Stx, Shiga toxin; ULN, upper limit of normal; WG, weeks of gestation.
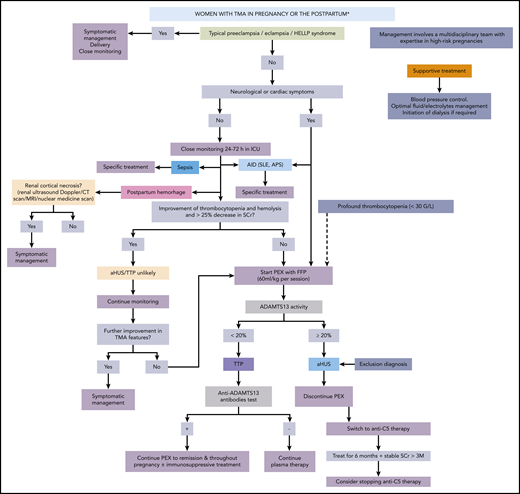
Algorithm for the treatment of TMA occurring during pregnancy and the postpartum period. The most likely diagnoses are shown in full colored rectangles. AID, autoimmune disease; FFP, fresh frozen plasma; ICU, intensive care unit; MRI, magnetic resonance imaging; PEX, plasma exchange; SCr, serum creatinine; SLE, systemic lupus erythematosus.
Algorithm for the treatment of TMA occurring during pregnancy and the postpartum period. The most likely diagnoses are shown in full colored rectangles. AID, autoimmune disease; FFP, fresh frozen plasma; ICU, intensive care unit; MRI, magnetic resonance imaging; PEX, plasma exchange; SCr, serum creatinine; SLE, systemic lupus erythematosus.
Some findings that may help in the clinical management of patients with P-TMA
| Findings to aid in management of patients with P-TMA . |
|---|
| 1. The context (PE/E, HELLP, severe delivery hemorrhage) in which TMA occurs is paramount. |
| 2. aHUS and TTP are rare disorders in general and during pregnancy.14,17,22,23 |
| 3. PE/E and HELLP syndrome are still the main cause of P-TMA.22,42 |
| 4. To date, there is no diagnostic test for aHUS and complement assays and results of genetic tests are not required for diagnosis at the acute phase |
| Normal complement assays do not rule out pregnancy-associated aHUS36,37 ; conversely, features of complement activation are not synonymous with pregnancy-associated aHUS (transient complement activation may be the consequence of endothelial damage). |
| 5. A pregnancy-associated aHUS or a TTP masquerading as HELLP is a very rare occurrence.26 |
| 6. Increased levels of serum liver enzymes are extremely rare in aHUS. |
| 7. The absence of thrombocytopenia does not rule out pregnancy-associated aHUS.23 |
| 8. HELLP syndrome is a TMA affecting mainly the liver and more rarely the kidney (the most frequent renal lesion is acute tubular necrosis).38,39 |
| 9. PE/E and HELLP syndrome are not predominantly complement-mediated TMA.41,109 |
| 10. Spontaneous evolution of renal/hematological parameters during the first 48 h after delivery is crucial in the management of P-TMA.42 |
| 11. Benefit of plasma exchanges is only proven in immune ADAMTS13-deficiency–related TTP. |
| 12. In case of anuria (particularly in context of postpartum hemorrhage), renal cortical necrosis (Doppler, magnetic resonance imaging) should be ruled out.40 |
| 13. A kidney biopsy, when feasible, may be helpful for the differential diagnosis between acute tubular necrosis, TMA, and other causes of AKI. |
| Findings to aid in management of patients with P-TMA . |
|---|
| 1. The context (PE/E, HELLP, severe delivery hemorrhage) in which TMA occurs is paramount. |
| 2. aHUS and TTP are rare disorders in general and during pregnancy.14,17,22,23 |
| 3. PE/E and HELLP syndrome are still the main cause of P-TMA.22,42 |
| 4. To date, there is no diagnostic test for aHUS and complement assays and results of genetic tests are not required for diagnosis at the acute phase |
| Normal complement assays do not rule out pregnancy-associated aHUS36,37 ; conversely, features of complement activation are not synonymous with pregnancy-associated aHUS (transient complement activation may be the consequence of endothelial damage). |
| 5. A pregnancy-associated aHUS or a TTP masquerading as HELLP is a very rare occurrence.26 |
| 6. Increased levels of serum liver enzymes are extremely rare in aHUS. |
| 7. The absence of thrombocytopenia does not rule out pregnancy-associated aHUS.23 |
| 8. HELLP syndrome is a TMA affecting mainly the liver and more rarely the kidney (the most frequent renal lesion is acute tubular necrosis).38,39 |
| 9. PE/E and HELLP syndrome are not predominantly complement-mediated TMA.41,109 |
| 10. Spontaneous evolution of renal/hematological parameters during the first 48 h after delivery is crucial in the management of P-TMA.42 |
| 11. Benefit of plasma exchanges is only proven in immune ADAMTS13-deficiency–related TTP. |
| 12. In case of anuria (particularly in context of postpartum hemorrhage), renal cortical necrosis (Doppler, magnetic resonance imaging) should be ruled out.40 |
| 13. A kidney biopsy, when feasible, may be helpful for the differential diagnosis between acute tubular necrosis, TMA, and other causes of AKI. |
P-TMA, pregnancy-associated TMA.
The diagnostic and to some extent therapeutic approach may be different in a patient with a first episode of pregnancy-associated TMA (requirement for an extensive diagnosis workup) as compared with a patient with a history of TTP, aHUS, or autoimmune disease who experiences TMA recurrence during pregnancy.
The approach to pregnancy-associated TMA has 2 main cornerstones. The first is to urgently rule in or out pregnancy-associated TTP with ADAMTS13 activity testing, due to the potential life-threatening complications of TTP. The second is to urgently diagnose pregnancy-associated complement-mediated aHUS in order to start specific treatment. To date, there is no reliable diagnostic test for aHUS (Figure 2; Case 2) and aHUS is diagnosed by exclusion, once all other diagnoses have been ruled out with a reasonable clinical probability.
Initial workup for pregnancy-associated TMA
This step-by-step procedure (Figure 2) aims to rule in or out all possible causes of pregnancy-associated TMA, including rare ones, as some require specific treatments. Testing of ADAMTS13 activity is of utmost importance as the diagnosis of TTP relies mostly, if not exclusively, on this test. However, urgent ADAMTS13 testing is not widely available, mostly for technical issues (biological methodology and expertise). In the absence of available assays, the PLASMIC and French scores that combine clinical and laboratory parameters (platelet count, <30 × 109/L; serum creatinine, <200 μmol/L; international normalized ratio, <1.3; median corpuscular volume, <86.5 fL; and hemolysis markers) may help predict ADAMTS13 deficiency.43,44 However, these scores have not been validated in the setting of pregnancy.
The initial workup should include the assessment of the soluble fms-like tyrosine kinase-1/placental growth factor (sFlt1/PlGF) ratio, a new useful tool to rule in/out hypertensive complications of pregnancy, increasingly used in obstetrics in several countries. When available, the sFlt-1/PlGF ratio should be measured at the diagnosis of pregnancy-associated TMA, before plasma therapy and delivery.
sFlt-1/PlGF ratios over 85 before 34 weeks of gestation, and over 110 after 34 weeks, are strongly suggestive of PE/eclampsia or HELLP syndrome, whereas ratios below 38 suggest an alternative diagnosis.45-47 However, further studies are required to confirm the diagnostic value of sFlt-1/PlGF ratio in pregnancy-associated TMA. Even though measurement of the sFlt-1/PlGF ratio is not rapidly available to guide the initial management, this ratio is important in retrospectively determining the etiology of pregnancy-associated TMA and in optimizing management of subsequent pregnancies. In all cases, in the absence of sFlt1/PlGF measurement, the diagnosis of PE/eclampsia and of HELLP syndrome still relies on simple clinical and laboratory parameters.
Positivity of autoimmunity tests should prompt multidisciplinary assessment of the presence of autoimmune disorders underlying TMA (mostly lupus and CAPS), which require urgent immunosuppressive and/or anticoagulant therapies.
In some instances, other rare diagnoses to be ruled in or out are: (a) Shiga-like toxin-producing Escherichia coli HUS (STEC-HUS48 ; polymerase chain reaction for Shiga toxin genes in stool); (b) cobalamin C deficiency-associated HUS, a rare form of TMA affecting not only children (mostly) but also young adults31 (measurement of plasma homocysteine levels and of methylmalonic acid levels in urine or plasma and ultimately homocystinuria type C protein [MMACHC] gene sequencing); and (c) TMA not related to pregnancy (Figure 2).
Finally, pregnancy-associated TMA may rarely be associated with renal cortical necrosis (RCN; irreversible ischemic necrosis of the renal cortex).49 A recent report from France40 reported a risk of RCN in patients with severe postpartum hemorrhage requiring the use of tranexamic acid.40 This risk is, however, low.50 Nevertheless, evaluating a patient for RCN in the setting of pregnancy-associated TMA complicated with anuria/severe oliguria is relevant in order to avoid unnecessary treatments, as hemolysis and thrombocytopenia usually abate spontaneously and no specific treatment can rescue renal function (Table 340,50-53 ).
Features of TMA associated with PPH complicated by RCN
| PPH and TMA . |
|---|
| 1. PPH (defined as blood loss >500 mL) may be associated with AKI resulting from hypovolemia, colloid infusion, or disseminated intravascular coagulation. |
| 2. More rarely, AKI-associated with PPH may have features of TMA (hemolytic anemia and severe thrombocytopenia). |
| In this context, especially in anuria/severe oliguria, RCN40 (irreversible ischemic necrosis of the renal cortex) should be ruled out (ideally using magnetic resonance imaging, or alternatively Doppler ultrasound) in order to avoid unnecessary treatments (plasma exchange, complement inhibitor). |
| 3. In a recent report of 18 cases of PPH complicated with RCN,40all patients had hemolytic anemia and AKI requiring dialysis. |
| Hemolysis and thrombocytopenia resolved within 7 d without specific treatment; 8 patients remained dialysis-dependent and 10 had chronic kidney disease. |
| 4. The potential role of a high dose (>2 g) and prolonged administration of tranexamic acid in the context of a “gravid endothelium” has been suggested.40 |
| (Tranexamic acid is an antifibrinolytic drug, which induces experimental TMA in rats).52 |
| 5. Nevertheless, PPH is a life-threatening complication of pregnancy and the WOMAN study53demonstrates a significant decrease of death due to bleeding in patients treated with tranexamic acid (first dose of 1 g with a possible second dose of 1 g, 30 min later) for PPH without renal side effects. |
| PPH and TMA . |
|---|
| 1. PPH (defined as blood loss >500 mL) may be associated with AKI resulting from hypovolemia, colloid infusion, or disseminated intravascular coagulation. |
| 2. More rarely, AKI-associated with PPH may have features of TMA (hemolytic anemia and severe thrombocytopenia). |
| In this context, especially in anuria/severe oliguria, RCN40 (irreversible ischemic necrosis of the renal cortex) should be ruled out (ideally using magnetic resonance imaging, or alternatively Doppler ultrasound) in order to avoid unnecessary treatments (plasma exchange, complement inhibitor). |
| 3. In a recent report of 18 cases of PPH complicated with RCN,40all patients had hemolytic anemia and AKI requiring dialysis. |
| Hemolysis and thrombocytopenia resolved within 7 d without specific treatment; 8 patients remained dialysis-dependent and 10 had chronic kidney disease. |
| 4. The potential role of a high dose (>2 g) and prolonged administration of tranexamic acid in the context of a “gravid endothelium” has been suggested.40 |
| (Tranexamic acid is an antifibrinolytic drug, which induces experimental TMA in rats).52 |
| 5. Nevertheless, PPH is a life-threatening complication of pregnancy and the WOMAN study53demonstrates a significant decrease of death due to bleeding in patients treated with tranexamic acid (first dose of 1 g with a possible second dose of 1 g, 30 min later) for PPH without renal side effects. |
PPH, postpartum hemorrhage.
When the aforementioned diagnoses have been ruled out, aHUS is diagnosed by exclusion (no positive diagnostic test available to date). Results of complement workup are not required for aHUS diagnosis and treatment (normal tests do not exclude the diagnosis of complement-mediated aHUS). Conversely, decreased levels of serum C3, C4, factor H, factor I and factor B, and/or increased soluble C5b-9 are not synonymous of aHUS and complement activation may be transient and self-limited in other forms of pregnancy-associated TMA.54 Nevertheless, complement workup is recommended in the initial phase of the management of pregnancy-associated TMA as:
(a) the detection of anti–factor H antibodies usually leads to the use of immunosuppressive treatments (cyclophosphamide, rituximab),
(b) the detection of a pathogenic complement variant retrospectively confirms the diagnosis of complement-mediated HUS, although, negative genetic tests do not rule out pregnancy-associated HUS,1,38,39
(c) genetics tests are helpful in the long-term for the decision of whether to discontinue anti-C5 treatment.55
Treatment of pregnancy-associated TMA
An algorithm for the treatment of pregnancy-associated TMA is shown in Figure 3 and illustrated in cases 1-3.
In most if not all cases, rapid delivery should be considered, as it may be sufficient to control some forms of pregnancy-associated TMA (PE/eclampsia, HELLP syndrome) or at least help achieve more rapid remission of other types of pregnancy-associated TMA (TTP and HUS). Even though vaginal delivery can be safely performed in intensive care unit (ICU) settings, it is not the usual mode of delivery in the context of pregnancy-associated TMA, especially when the disease is diagnosed before 37 weeks of gestation.56 In practice, most women with severe maternal complications have delivery by caesarean section (80% in patients with HELLP syndrome57 ). During the initial management of pregnancy-associated TMA, and after initial blood testing (especially sFlt-1/PlGF and complement and ADAMTS-13 tests), plasma exchange should be initiated in the following situations:
(a) at TMA diagnosis in a patient with atypical presentation of PE/eclampsia or HELLP syndrome, and life-threatening neurological (seizures, altered consciousness, coma) or cardiac (elevated troponin levels on systematic screening, electrocardiographic abnormalities, altered cardiac function) signs and potentially profound thrombocytopenia (<30 g/L): plasma exchange should be performed as for acute TTP58-60 until ADAMTS13 tests results are available.
(b) In all other cases, after 24 to 72 hours of close monitoring, if improvement of thrombocytopenia and hemolysis and >25% decrease in serum creatinine do not occur. During this initial phase (4-5 days) of plasma exchange, differential diagnosis is completed and plasma exchange is used to treat a potential TTP. Once diagnostic workup is completed, plasma exchange is continued only if a diagnosis of TTP is made (Case 1) (and in the rare occurrence of CAPS). If aHUS diagnosis is made by exclusion of all alternative diagnoses, plasma exchange is discontinued and patients are switched to anti-C5 treatment (Case 2).
Treatment of pregnancy-associated HUS
Traditionally, pregnancy-associated HUS was managed by plasma exchange and/or plasma infusions.2,23 However, plasma treatment was shown to be effective in only one-half of patients; in a retrospective study, the risk of end-stage renal disease was similarly high (∼50%) in the 56 women who received plasma exchange vs the 16 who did not.23 The advent of the humanized monoclonal anti-C5 antibody (eculizumab) has radically improved the prognosis of aHUS.61 Its use appears to be safe for both mother and fetus in pregnancy, as documented in women with paroxysmal nocturnal hemoglobinuria62 and aHUS (supplemental Table 223,24,55,63-87 ), even though the drug was detected in one-third of cord blood samples.62
No controlled clinical trials have been performed with anti-C5 treatment in pregnancy-associated HUS; however, >35 patients who received anti-C5 treatment of HUS during pregnancy or postpartum are reported in the literature (supplemental Table 2). Approximately 90% of patients underwent hematological and renal remission, but this proportion may be overestimated due to publication bias in favor of successful outcomes. In a retrospective study of 22 women who developed pregnancy-associated HUS, all 10 patients who received anti-C5 treatment underwent hematological and renal remission.24 Of note, in 9 of the 10 cases, anti-C5 treatment was initiated because of the lack of response to plasma infusions or exchanges. None of the patients who received anti-C5 treatment, and 6 of 12 patients (50%) who did not, reached end-stage renal disease at the end of 2-year follow-up.
Thus, patients who are assumed, based on a reasonable clinical probability, to have pregnancy-associated HUS should receive anti-C5 treatment. Careful monitoring of the degree of terminal complement blockade is required because pregnancy may require an increased dose and/or frequency of infusions due to higher distribution volume, increased C5 synthesis,25 and/or urinary loss in case of heavy proteinuria.
Duration of long-term treatment is unclear and the decision should be individualized. The presence of complement gene abnormalities is a risk for aHUS relapse after anti-C5 treatment discontinuation,88 including in patients with pregnancy-associated HUS (supplemental Table 2).
Treatment of TTP occurring during pregnancy
Treatment of pregnancy-associated TTP yields a complete response rate of ∼80% to 90%, similar to that observed globally in TTP.14,54,60 However, the combination of TTP and PE/HELLP syndrome significantly increases maternal mortality (up to 44%, albeit in old studies),58 and maternal outcome has improved with the amelioration of treatment modalities.
In contrast to PE/eclampsia and HELLP syndrome, which improve following delivery, in TTP, delivery is recommended only for those women who do not respond to plasma exchange or in whom there is fetal compromise.
Finally, platelet transfusions, which may be required for obstetric procedures (eg, caesarean section) or central catheter insertion prior to plasma exchange, should be avoided in patients with TTP due to concerns regarding thrombosis and worsening of neurological symptoms.89,90 If platelet transfusion is urgently required, it should be administered along with plasma exchange.
First episode of pregnancy-associated immune TTP.
The standard treatment of immune TTP during pregnancy is plasma exchange (1.0-1.5 plasma volume, 40-60 mL/kg fresh-frozen plasma), with steroids, either as a pulsed dose of methylprednisolone or oral therapy (1 mg/kg per day with tapering over 2-3 months). In case the positive results of anti-ADAMTS13 antibodies are available before delivery, the use of immunosuppressive drugs is limited to azathioprine, calcineurin inhibitors, and rituximab. The use of rituximab in pregnancy-associated TTP has been reported in very rare patients91,92 with no documented maternal or fetal toxicity. However, a review of rituximab use in 231 pregnancies confirmed its ability to cross the placenta93 via active transport (placental Fc receptors) especially by the end of the third trimester.94 Besides, hematologic abnormalities or malformations have been reported in a series of 90 live births after rituximab use in pregnancy (including 3 maternal TTP cases), prompting caution in its use.93 Thus, data supporting the use of rituximab in pregnancy are currently limited, and other therapies are preferred. However, refractory immune TTP not responding to treatment, or evidence of maternal or fetal compromise, may necessitate delivery, allowing for the use of further immunosuppressive therapy, including rituximab.
To date, no data are available regarding the use during pregnancy of caplacizumab, a monoclonal nanobody directed against VWF, and this therapy should not be used in pregnancy.
First episode of pregnancy-associated hereditary TTP.
The diagnosis of hereditary TTP is unlikely to be made during the initial phase of the disease; therefore, patients most frequently undergo plasma exchange as for immune TTP, and repeat ADAMTS 13 testing and mutational analysis are usually available after achieving TTP remission with plasma exchanges. Rapid normalization of platelet count with plasma exchange may suggest the absence of anti-ADAMTS 13 antibodies (with undetectable ADAMTS13 activity).
Counseling and management of a patient with a history of aHUS, TTP, and PE/eclampsia/HELLP who wishes to start a pregnancy
This counseling requires a multidisciplinary assessment and provision to the patient of full information on the maternal (potential TMA recurrence and hypertensive complications of pregnancy) and fetal (preterm delivery, fetal growth restriction, and perinatal death) risks inherent in this high-risk pregnancy.
Of note, there is no consensus regarding the prophylactic use of low-dose aspirin in women with a history of aHUS or TTP, which is not one of the criteria for aspirin prophylactic use in national guidelines.95-98 Nevertheless, these diseases are rare and a rather liberal prophylactic use of low-dose aspirin (started prior to 15 weeks of gestation) is to be discussed on a case-by-case basis.
Patient with a history of aHUS
Currently, pregnancy is not contraindicated in women with a history of aHUS (Table 415,18,61,63,64 ). First, the risk of relapse of aHUS during pregnancy or postpartum appears lower (∼25%) than formerly believed.61 Second, an effective specific treatment has become available. In the vast majority of aHUS cases, anti-C5 treatment reverses AKI,88 so women planning a pregnancy after an episode of aHUS usually have normal or near-normal renal function. Moreover, in the case of aHUS recurrence during pregnancy, prompt anti-C5 treatment optimizes the patient’s chance of a full recovery and a term live birth. Currently, prophylactic anti-C5 treatment is not recommended. Close clinical and laboratory monitoring is empirically sound practice until 3 to 4 months postdelivery.
Helpful elements for counseling a patient with a history of aHUS who wishes to plan a pregnancy
| Counseling a woman with a history of aHUS about pregnancy relies on the following information: . |
|---|
| 1. Pregnancy is no longer contraindicated in women with a history of aHUS. |
| The risk of relapse of aHUS during pregnancy or postpartum appears lower (∼25%) than formerly appreciated.83 |
| An efficient treatment (anti-C5 treatment such as eculizumab) is available. |
| 2. The risk of relapse of aHUS triggered by pregnancy is difficult to predict. |
| A prior uneventful pregnancy does not guarantee subsequent pregnancies will be free of relapse.21,83 |
| Women who do not carry a complement gene variant are not protected from pregnancy aHUS.21 |
| 3. An interval of ∼12 mo of aHUS remission and stabilized renal function is appropriate before pregnancy initiation. |
| 4. In women with prior aHUS, relapse of aHUS occurs more frequently during pregnancy than after delivery.21,23 |
| In the pre-anti-C5 treatment era, this was associated with a high risk of fetal death or preterm birth.83 |
| 5. CKD may be a limitation to pregnancy. |
| Residual severe CKD or hypertension after aHUS may worsen during pregnancy, with increased risk of preeclampsia or HELLP syndrome, ESRD, and fetal death.24,83 |
| 6. In case of aHUS relapse, prompt anti-C5 treatment initiation optimizes chances of patient’s full recovery and child’s full-term live birth. |
| 7. Prophylactic anti-C5 treatment is currently not recommended. |
| Anti-C5 treatment is usually not discontinued in women already treated prior to pregnancy (particularly renal transplant patients). |
| 8. Pregnancy in a woman with a history of aHUS remains a high-risk pregnancy. |
| Close multidisciplinary (obstetricians, nephrologists, neonatologists, and complement biologists) supervision from the first weeks of pregnancy and up to 3 mo postdelivery in high-risk pregnancy maternity clinic is mandatory. |
| Counseling a woman with a history of aHUS about pregnancy relies on the following information: . |
|---|
| 1. Pregnancy is no longer contraindicated in women with a history of aHUS. |
| The risk of relapse of aHUS during pregnancy or postpartum appears lower (∼25%) than formerly appreciated.83 |
| An efficient treatment (anti-C5 treatment such as eculizumab) is available. |
| 2. The risk of relapse of aHUS triggered by pregnancy is difficult to predict. |
| A prior uneventful pregnancy does not guarantee subsequent pregnancies will be free of relapse.21,83 |
| Women who do not carry a complement gene variant are not protected from pregnancy aHUS.21 |
| 3. An interval of ∼12 mo of aHUS remission and stabilized renal function is appropriate before pregnancy initiation. |
| 4. In women with prior aHUS, relapse of aHUS occurs more frequently during pregnancy than after delivery.21,23 |
| In the pre-anti-C5 treatment era, this was associated with a high risk of fetal death or preterm birth.83 |
| 5. CKD may be a limitation to pregnancy. |
| Residual severe CKD or hypertension after aHUS may worsen during pregnancy, with increased risk of preeclampsia or HELLP syndrome, ESRD, and fetal death.24,83 |
| 6. In case of aHUS relapse, prompt anti-C5 treatment initiation optimizes chances of patient’s full recovery and child’s full-term live birth. |
| 7. Prophylactic anti-C5 treatment is currently not recommended. |
| Anti-C5 treatment is usually not discontinued in women already treated prior to pregnancy (particularly renal transplant patients). |
| 8. Pregnancy in a woman with a history of aHUS remains a high-risk pregnancy. |
| Close multidisciplinary (obstetricians, nephrologists, neonatologists, and complement biologists) supervision from the first weeks of pregnancy and up to 3 mo postdelivery in high-risk pregnancy maternity clinic is mandatory. |
CKD, chronic kidney disease; ESRD, end-stage renal disease.
Nevertheless, pregnancy in women with a history of aHUS remains high risk, and close supervision is required from the first weeks of pregnancy up until 3 to 4 months postdelivery. Follow-up should include: (1) close ambulatory monitoring of blood pressure and urinary dipsticks (at least twice weekly); (2) frequent multidisciplinary visits (at least every 4-6 weeks); (3) periodic laboratory tests: serum creatinine, urinary protein, liver enzymes, hemolysis parameters, sFlt1/PlGF (when available); and (4) obstetric ultrasound evaluation that usually includes 20-week uterine artery Doppler, and subsequent 4-weekly biometry and fetal Doppler scans. However, the cost-effectiveness of these evaluations remains to be assessed.
Women should be informed of the estimated ∼25% risk of aHUS recurrence. They should also be informed of the potential maternal (renal function deterioration, PE/eclampsia, HELLP) and fetal (preterm birth, intrauterine death) risks due to subclinical or clinical chronic renal disease even in its early stages.99,100 In some cases, significant preexisting renal impairment, rather than the risk of aHUS relapse, may be a limitation for pregnancy. A history of renal transplantation further increases the risk.
It is noteworthy that, although some animal101,102 and clinical103,104 data have suggested a link between PE/eclampsia and complement activation, in clinical practice, anti-C5 treatment does not fully prevent PE/eclampsia in patients with aHUS25 or with paroxysmal nocturnal hemoglobinuria.62
Genetic counseling regarding the risk of aHUS in the offspring of a patient with a pathogenic complement variant should take into account the fact that such variants are only risk factors for aHUS and thus penetrance of the disease is not 100%. A large proportion of relatives of a patient with aHUS who carries a pathogenic complement gene variant are usually unaffected by aHUS. Screening for complement gene variants in a healthy woman planning pregnancy, who has a relative with a history of aHUS, is to be discussed on a case-by-case basis depending on local practice and legal framework for genetic testing. In the case of a pathogenic variant detected in an otherwise healthy woman, close monitoring during pregnancy and the postpartum is warranted.
Patient with a history of TTP
Patient with immune TTP
In women recovered from immune TTP, the precise risk of relapse during pregnancy is unknown. This risk mainly depends on the level of ADAMTS13 activity at pregnancy onset (increased risk if activity remains undetectable). Serial monitoring, at least in each trimester and in the postpartum period, of ADAMTS13 activity in all immune TTP pregnant women is highly recommended.91 However, its cost-effectiveness remains to be fully assessed. Reported experience is scarce and preemptive strategies in this context are still highly empirical.
The patient should be informed that a severe reduction in ADAMTS13 to <20% would be an indication for elective therapy to raise the enzyme level and prevent TTP relapse. She should also be informed of the side effects of potential therapies required: steroids, azathioprine, calcineurin inhibitors, and plasma exchange: these therapies are preferred to rituximab.
Patient with hereditary TTP.
In hereditary TTP, the risk of relapse in the subsequent pregnancy is 100% in the absence of plasma prophylaxis.105 Thus, prophylactic plasma infusions should be initiated at ∼10 weeks of gestation, or immediately after the diagnosis of pregnancy, and continued throughout pregnancy and postpartum. Empirically, a volume of 10 mL/kg to 15 mL/kg plasma every 10 to 21 days is usually recommended. This regimen needs to be adapted to prevent cytopenia and hemolysis. Typically, the volume of plasma and the frequency of administration increase from ∼20 weeks of gestation; in the third trimester, therapeutic plasma exchange may be required to prevent fluid overload.14,54 Treatment aims to maintain a normal platelet count, to prevent hemolysis, and, ideally, to restore ADAMTS13 activity >20%. Delivery is usually induced at ∼37 weeks. Prophylactic plasma infusions in hereditary TTP result in an excellent fetal and maternal prognosis, by limiting placental micro-occlusion arterial thrombosis.14,54
Women with hereditary TTP should be informed of the need for early start of prophylactic plasma infusions and for close monitoring with serial growth scans and determination of uterine artery flow. Hereditary TTP is an autosomal-recessive disease and thus the risk of inheritance in the offspring of an affected patient is extremely unlikely, except in consanguineous families.
Patient with a history of PE/eclampsia/HELLP
Reported recurrence rates in the literature range from a few percent up to 65%. Recently, an individual patient data meta-analysis including a total of 99 415 women reported a recurrence risk of 21% of hypertensive disorders and 14% of recurrent PE. After a previous HELLP syndrome, the recurrence rate is 18% for PE and 7% for HELLP syndrome.106 Prevention of this recurrence is based on low-dose aspirin (75-150 mg per day) once a day in the evening, started before 16 weeks of gestation.5
Conclusion
New advances in the management of pregnancy-associated HUS and pregnancy associated-TTP have clearly improved the prospects for pregnancy in patients affected by these forms of TMA. Pregnancy in these patients remains high risk, and providing objective information to the woman on potential risks and complications, combined with close multidisciplinary monitoring throughout pregnancy and postpartum, is paramount. The clinical guidance presented in this report will probably evolve in the near future with the accumulation of new data on pregnancy-associated TMA and the availability of new treatments (new complement inhibitors, caplacizumab, recombinant ADAMTS13, etc). The design of an international registry of pregnancy-associated TMA will also help to improve our knowledge of these severe complications of pregnancy.
The online version of this article contains a data supplement.
Acknowledgments
The authors are grateful to David Buob (Department of Pathology, Hôpital Tenon, Paris, France) for providing the picture of the kidney biopsy section shown in Figure 1.
M.N. was supported by Italian Ministero della Salute grants RF-2016-02361720 and CC-2015-2361053.
The authors have received no direct or indirect financial support of any kind for the design or the writing of the present consensus document.
Authorship
Contribution: All authors contributed to the discussions leading to the consensus paper; F.F., M.S., P.C., C.L., M.N., and G.R. drafted the paper; and all authors reviewed and approved the paper.
Conflict-of-interest disclosure: F.F. has received consultancy and/or speaker honoraria from Roche, Alexion, Apellis, Achillion, Novartis, and Alnylam. M.S. has received speaker fees and advisory board honoraria from Alexion, Sanofi, Novartis, and Takeda, and a research grant from Shire. M.B. has received honoraria for lectures and advisory boards from Alexion. F. Provôt has received speaker fees from Alexion and Sanofi. D.K. is a director of, and scientific advisor to, Gyroscope Therapeutics, and has received honoraria for consultancy work from Alexion, Idorsia, Apellis, and Novartis. K.P. has received fees as a speaker and advisory panel member for Alexion and UCB Australia. F. Pène has received speaker fees (institutional contract) from Alexion. A.H. has received speaker honoraria from Alexion. P.C. has received fees for advisory board membership from Sanofi, Alexion, and Takeda, and research grants from Sanofi, Alexion, and Roche. Y.D. has received lecture fees from Alexion, and honoraria for advisory board membership from Sanofi-Ablynx. L.A. has received research funding and/or financial support for the Centre Hospitalier Universitaire Vaudois hemophilia program and/or speaker honoraria and/or consultancy fees for advisory boards and/or travel reimbursement from Bayer, Boehringer Ingelheim, Daiichi Sankyo, CSL-Behring, Novartis, NovoNordisk, Octapharma, OrPha Swiss, Pfizer, Roche, Sanofi-Aventis, Sanofi-Genzyme, Siemens, Shire/Takeda, and Sobi (no personal remuneration is accepted; compensation is paid to the institution for research and educational activities). A.V. has received fees as a member of an advisory board (caplacizumab) from Ablynx-Sanofi. V.F.-B. has received fees from Alexion Pharmaceuticals, Roche, and Apellis for invited lectures and/or board membership, and is the recipient of a research grant from Alexion Pharmaceuticals. M.N. has received honoraria from Alexion Pharmaceuticals for giving lectures, and research grants from Omeros, Roche, and Alnylam. S.C, has received research funding and consulting fees from Sanofi Genzyme, consulting and advisory board fees from Alexion, consulting fees from Regeron, and research funding and consulting fees from Takeda. C.L. has received consultancy fees for advisory boards from Alexion, Roche, and Inmunova. G.R. has received speaker honoraria and/or travel reimbursements and/or consultancy fees for advisory boards from Alnylam, Boehringer Ingelheim, Handok Inc, Inception Sciences Canada, Alexion Pharmaceuticals Inc, Janssen Pharmaceutical, and Achillion Pharmaceuticals (no personal remuneration is accepted; compensation is paid to the institution for research and educational activities). VT has received consultancy and/or speaker honoraria from Roche Diagnostics, Alexion, and Obseva. The remaining authors declare no competing financial interests.
A complete list of the members of The International Working Group on Pregnancy-Related Thrombotic Microangiopathies appears in the supplemental appendix.
Correspondence: Fadi Fakhouri, Service of Nephrology and Hypertension, Department of Medicine, Lausanne University Hospital, Rue du Bugnon 17, 1005 Lausanne, Switzerland; e-mail: fadi.fakhouri@unil.ch.