Key Points
Extensive antithrombin and heparin prophylaxis did not completely prevent the risk of VTE.
Fibrinogen concentrates increase the risk of thrombosis and should be restricted to patients with hemorrhage.
Abstract
Patients undergoing treatment of acute lymphoblastic leukemia (ALL) are at risk for thrombosis, caused in part by the use of l-asparaginase (L-ASP). Antithrombin (AT) replacement has been suggested to prevent venous thromboembolism (VTE) and thus may increase exposure to ASP. We report herein the results of the prophylactic replacement strategy in the pediatrics-inspired prospective GRAALL-2005 study. Between 2006 and 2014, 784 adult patients with newly diagnosed Philadelphia− ALL were included. The incidence rate of VTE was 16%, with 69% of cases occurring during induction therapy. Most patients received AT supplementation (87%). After excluding patients who did not receive L-ASP or who developed thrombosis before L-ASP, AT supplementation did not have a significant impact on VTE. Administration of fibrinogen concentrates was associated with an increased risk of VTE, whereas transfusion of fresh frozen plasma had no effect. Heparin prophylaxis was associated with an increased risk of VTE. Prophylactic measures were not associated with an increased risk of grade 3 to 4 bleeding complications. The rate of VTE recurrence after L-ASP reintroduction was 3% (1 of 34). In ALL patients receiving L-ASP therapy, the use of fibrinogen concentrates may increase the risk of thrombosis and should be restricted to rare patients with hypofibrinogenemia-induced hemorrhage. VTE developed despite extensive AT supplementation, which suggests the need for additional prophylactic measures. Although this large descriptive study was not powered to demonstrate the efficacy of these prophylactic measures, it provides important insight to guide future trial design. This trial was registered at www.clinicaltrials.gov as #NCT00327678.
Medscape Continuing Medical Education online
In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 373.
Disclosures
Author Corentin Orvain received honoraria from Incyte and Novartis Pharmaceuticals and author Mathilde Hunault-Berger served as an advisor or consultant for Erytech and received honoraria from Jazz Pharmaceuticals, Inc. Editor Nancy Berliner, CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, and the remaining authors declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe the incidence of venous thromboembolism (VTE) among adult patients with acute lymphoblastic leukemia (ALL) included in the pediatrics-inspired GRAALL-2005 protocol
Identify demographics- and disease-related factors associated with VTE among adult patients with ALL
Determine the efficacy and safety of the prophylactic strategy for VTE used in the pediatrics-inspired GRAALL-2005 protocol
Release date: July 16, 2020; Expiration date: July 16, 2021
Introduction
The prognosis of adult patients with acute lymphoblastic leukemia (ALL) has greatly improved since the introduction of pediatrics-inspired protocols such as those developed by the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL).1-4 l-Asparaginase (L-ASP) has a key role in these regimens.5 This enzyme catalyzes the hydrolysis of l-asparagine, resulting in its depletion. Because of the low levels of l-asparagine synthetase, lymphoblasts are particularly dependent on external sources of l-asparagine for protein synthesis and cell surival.6,7 Patients who undergo treatment of ALL are at risk of thrombosis caused by the disease itself, by the use of steroids, but also by the use of L-ASP.8-12 Indeed, L-ASP inhibits the hepatic synthesis of l-asparagine–dependent hemostatic proteins, such as antithrombin (AT), and may induce endothelium activation.10-13 Thrombotic events were found to occur during induction therapy at a rate of 4.8% in 1280 children included in 17 prospective studies and of 5.9% in 323 adults included in 13 prospective studies.14,15 These events are responsible for thrombosis-related deaths or disabilities, delayed treatment, and bleeding complications from anticoagulation therapy, which requires more platelet transfusions to be safely administered.16
In addition, previous reports showed that patients with venous thromboembolism (VTE) have a lower event-free survival (EFS) that may be related to early discontinuation of L-ASP.16-18 The prophylactic infusion of fresh frozen plasma (FFP) has shown contradictory effects on the prevention of VTE.17-20 The repletion of L-ASP with FFP may counteract the action of L-ASP,21 but it has been suggested that AT replacement could decrease the rate of thrombosis and thus prevent L-ASP discontinuation.18,22-25 The GRAALL group has introduced prophylactic measures for thromboembolic and bleeding complications, such as infusion of FFP or fibrinogen concentrates in cases of hypofibrinogenemia and AT concentrates in cases with low AT levels. Although the GRAALL-2005 study was not powered to demonstrate the efficacy of this prophylactic strategy, compliance with these preventive measures was prospectively recorded to gain insight into thrombotic and bleeding complications.
The objectives of this study were (1) to evaluate the incidence of VTE among adult patients included in the pediatrics-inspired GRAALL-2005 protocol; (2) to identify demographic- and disease-related factors associated with VTE; and (3) to evaluate the impact of the prophylactic strategy used in our protocol.
Patients and methods
Patients and setting
The GRAALL-2005 trial was conducted from 2006 to 2014 at 57 French, 8 Belgian, and 8 Swiss centers. This randomized trial evaluated the impact of high-dose cyclophosphamide and rituximab during induction therapy in adult patients aged 18 to 65 years, with ALL without BCR-ABL.4,26 In brief, all patients received 5-drug induction therapy with 8 native Escherichia coli L-ASP (E coli L-ASP; Kidrolase) IV injections (6000 IU/m2 per injection) on days 8, 10, 12, 20, 22, 24, 26, and 28. A single methotrexate intrathecal (IT) injection was administered during the steroid prephase followed by 2 additional triple IT injections on days 1 and 8. In cases with central nervous system (CNS) involvement, 2 triple IT injections per week were administered for a total of 8 injections, then 1 injection per week, for a total of 12 injections. Patients in complete remission (CR) received 2 consolidation courses of 3 alternating blocks with high-dose methotrexate, high-dose aracytine, and cyclophosphamide. Each consolidation course contained 2 E coli L-ASP IV injections (10 000 IU/m2 per injection). High-risk patients were defined by ≥1 of the following criteria: CNS involvement; a white blood cell (WBC) count of 30 × 109/L or higher; a CD10− immature immunophenotype; poor cytogenetic abnormalities; poor early peripheral blood (PB) blast clearance, defined by a PB blast count >1.0 × 109/L at the end of the prephase; poor early bone marrow blast clearance, defined by >5% blasts in the bone marrow on day 8 of first induction; or late CR, defined by a need for salvage reinduction to achieve CR.26 High-risk patients ≤55 years were eligible for allogeneic stem cell transplantation in the first CR. Patients in persistent CR not eligible for allogeneic stem cell transplantation received a late intensification with the same drugs as in the induction course (with reduced anthracycline doses on days 15 and 16), followed by the repetition of 1 consolidation course. The full GRAALL 2005 protocol is shown in supplemental Figure 1, available on the Blood Web site. Informed consent was obtained from all patients at trial entry. The trial was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee (Ile-de-France VI, France).
Recommendations for thrombosis and bleeding prophylaxis were based on the CAPELAL study results.18 AT and fibrinogen levels were evaluated prospectively every other day from days 8 to 16 and from days 20 to 28 when patients were receiving L-ASP during induction and late intensification. FFP or fibrinogen concentrates were recommended if fibrinogen levels were <0.5 g/L, platelet transfusion support was recommended for platelets <20 × 109/L, and AT concentrate substitution therapy (Aclotine or Kybermin, 25 IU/kg) was recommended to maintain AT levels >60%. Unfractionated heparin at 100 IU/kg per day via continuous infusion was recommended during induction; low molecular weight heparin (LMWH) at prophylactic doses in subcutaneous injection could be used as replacement at the physician’s discretion. Heparin prophylaxis was recommended from day 1 of induction until the end of induction. When platelets were <20 × 109/L, heparin prophylaxis was interrupted, and the patient received a platelet transfusion. Heparin prophylaxis was resumed after platelet transfusion. In accordance with standard recommendations at that time, E coli L-ASP was replaced with Erwinia chrysanthemi L-ASP (Erwinase) 12 000 IU/m2 injections in cases of clinical hypersensitivity reactions.27 Nomegestrol was recommended for use as a contraceptive in women of childbearing age.
Data collection
The occurrence of any thrombosis in patients included in the GRAALL-2005 protocol was prospectively reported to the GRAALL data management center as serious adverse events. All cases of VTE were identified prospectively by clinical signs and confirmed by radiological imaging based on institutional guidelines. Patients were not screened for asymptomatic TE and superficial thrombophlebitis was not registered as VTE. Diffuse intravascular coagulation (DIC) was evaluated according to international guidelines.28
Statistical analysis
Categorical variables were presented as numbers with proportions and compared using the χ2 test or Fisher’s exact test, for small samples (expected values <5). Continuous variables were presented as medians with interquartile range (IQR) and compared using the nonparametric Mann-Whitney U test and Kruskal-Wallis test, as appropriate. Risk factors for VTE and the impact of prophylactic measures on VTE during induction and late intensification were analyzed by univariable logistic regression analysis. All factors with statistically significant associations (P < .1) were included in a multivariable logistic regression model. Results were all expressed as odds ratio (OR) with 95% confidence intervals (95% CI). The Kaplan-Meier method was used to estimate median EFS (failure to achieve CR, relapse, and death) and overall survival (OS, death) and survival curves were compared by using the log-rank test. All tests were 2-sided with a significance level of P ≤ .05. Statistical analysis was performed with SPSS software version 20 (SPSS Inc, Chicago, IL).
Results
Incidence of thrombosis
From 2006 to 2014, 813 adult patients with newly diagnosed Philadelphia− ALL were randomized. Twenty-nine patients were excluded by noneligibility criteria (11 patients), loss to follow-up (12 patients), lack of data regarding toxicity (3 patients), and withdrawal of consent (3 patients). Therefore, 784 patients were included in the study.
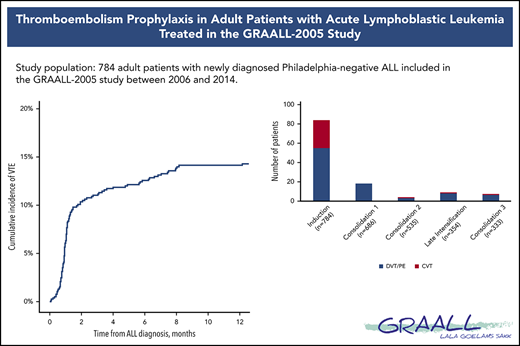
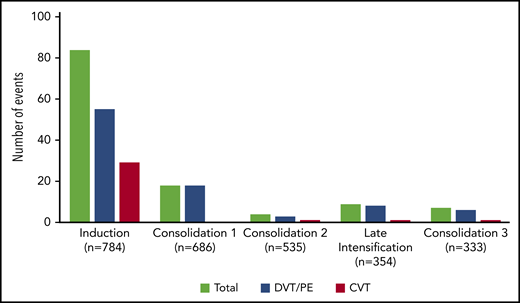
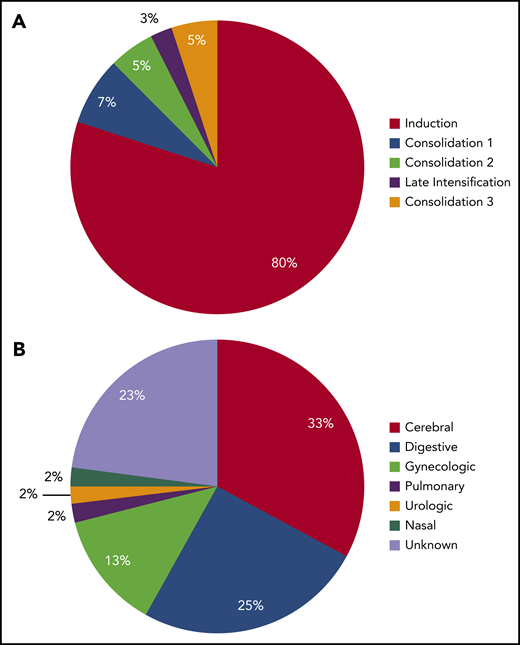
The incidence rate of VTE was 16% (122 VTEs in 112 patients) during the intensive part of chemotherapy (induction, consolidations, and late intensification). The 1-year cumulative incidence of first VTE was 17% (112 events in 658 patient-years; supplemental Figure 2). VTEs included 75 (62%) deep vein thromboses (DVTs), with 25 (33%) in the lower limb and 50 (67%) in the upper limb; 32 (26%) cerebral venous thromboses (CVTs); and 15 (12%) pulmonary embolisms (PEs). All VTEs were symptomatic, including central line–related thrombosis. Eighty-four (69%) VTEs occurred during induction therapy, with 6 patients having more than 1 thrombosis. Other VTEs occurred as follows: 18 (15%) during consolidation phase 1, 4 (3%) during consolidation phase 2, 9 (7%) during late intensification, and 7 (6%) during consolidation phase 3 (Figure 1). The type of thrombosis was different per treatment phase, as most CVTs occurred during induction therapy (29 during induction vs 3 during subsequent phases of treatment; P = .003).
Number of thrombosis per treatment phase. Seven patients developed VTE at different sites (3 patients with DVT and CVT, 2 with PE and CVT, 1 with DVT and PE during induction therapy, and 1 with DVT and PE during consolidation), and 3 patients with DVT experienced relapse of DVT during a subsequent phase of treatment.
Number of thrombosis per treatment phase. Seven patients developed VTE at different sites (3 patients with DVT and CVT, 2 with PE and CVT, 1 with DVT and PE during induction therapy, and 1 with DVT and PE during consolidation), and 3 patients with DVT experienced relapse of DVT during a subsequent phase of treatment.
Risk factors for thrombosis
Patient characteristics are described in Table 1. In our cohort, risk factors at diagnosis for thrombosis included older age, female sex, high body mass index (BMI), and a high platelet count at diagnosis (Table 2). After multivariable analysis, only platelet count at diagnosis was associated with an increased global risk of thrombosis (OR, 1.02; 95% CI, 1.00-1.04; P = .02). Morbid obesity (BMI >40 kg/m2) was present in only 8 patients and not associated with an increased risk of thrombosis. Risk factors were different according to the type of VTE: DVT/PE or CVT. Age (OR, 1.23; 95% CI, 1.03-1.48; P = .02), a high BMI (OR, 1.05; 95% CI, 1.00-1.10; P = .03), and a high platelet count at diagnosis (OR, 1.03; 95% CI, 1.00-1.05; P = .03) were associated with an increased rate of DVT/PE after multivariable analysis. In detail, age >25 years, a BMI >25 kg/m2, and a platelet count >200 × 109/L were associated with an increased risk of DVT/PE (supplemental Figure 3). In contrast, the only risk factor for CVT was a high hemoglobin level at diagnosis (OR, 1.17; 95% CI, 1.03-1.33; P = .01; Tables 1 and 2).
Characteristics of the whole cohort and of patients with DVT/PE and with CVT
| . | All (n = 784) . | Any thrombosis (n = 112) . | DVT/PE (n = 85) . | CVT (n = 32) . |
|---|---|---|---|---|
| Age, median (IQR), y | 36 (25-48) | 39 (28-51) | 39 (28-51) | 38 (23-43) |
| Female, n (%) | 312 (40) | 54 (48) | 39 (46) | 16 (50) |
| BMI, median (IQR), kg/m2 | 24 (21-27) | 25 (22-28) | 25 (22-28) | 23 (21-26) |
| Smoking, n (%) | ||||
| No | 396 (50) | 63 (56) | 49 (58) | 16 (50) |
| Yes | 226 (29) | 28 (25) | 21 (25) | 10 (31) |
| Missing data | 162 (21) | 21 (19) | 15 (17) | 6 (19) |
| Contraception, n (%) | ||||
| No | 124 (16) | 24 (21) | 17 (20) | 8 (25) |
| Yes | 78 (10) | 13 (12) | 10 (12) | 3 (9) |
| Missing data | 110 (14) | 17 (15) | 12 (14) | 5 (16) |
| Not applicable | 472 (60) | 58 (52) | 46 (54) | 16 (50) |
| Phenotype, n (%) | ||||
| B-cell | 523 (67) | 70 (63) | 52 (61) | 20 (63) |
| T-cell | 261 (33) | 42 (37) | 33 (39) | 12 (38) |
| CNS involvement, n (%) | 55 (7) | 5 (5) | 3 (4) | 2 (6) |
| WBC count at diagnosis, median (IQR), G/l | 12 (4-42) | 13 (4-47) | 13 (4-49) | 14 (3-51) |
| Hemoglobin level at diagnosis, median (IQR), g/dL | 10 (8-12) | 11 (9-13) | 10 (8-12) | 12 (10-14) |
| Platelet count at diagnosis, median (IQR), G/l | 72 (32-154) | 89 (40-196) | 94 (32-207) | 84 (56-167) |
| Prothrombin ratio at diagnosis, median (IQR) | 82 (72-92) | 85 (73-92) | 85 (74-92) | 85 (71-90) |
| Fibrinogen level at diagnosis, median (IQR), g/L | 4 (3-5) | 4 (3-5) | 4 (3-6) | 4 (2-6) |
| Poor early PB blast clearance, n (%) | 184 (24) | 19 (17) | 15 (18) | 4 (13) |
| . | All (n = 784) . | Any thrombosis (n = 112) . | DVT/PE (n = 85) . | CVT (n = 32) . |
|---|---|---|---|---|
| Age, median (IQR), y | 36 (25-48) | 39 (28-51) | 39 (28-51) | 38 (23-43) |
| Female, n (%) | 312 (40) | 54 (48) | 39 (46) | 16 (50) |
| BMI, median (IQR), kg/m2 | 24 (21-27) | 25 (22-28) | 25 (22-28) | 23 (21-26) |
| Smoking, n (%) | ||||
| No | 396 (50) | 63 (56) | 49 (58) | 16 (50) |
| Yes | 226 (29) | 28 (25) | 21 (25) | 10 (31) |
| Missing data | 162 (21) | 21 (19) | 15 (17) | 6 (19) |
| Contraception, n (%) | ||||
| No | 124 (16) | 24 (21) | 17 (20) | 8 (25) |
| Yes | 78 (10) | 13 (12) | 10 (12) | 3 (9) |
| Missing data | 110 (14) | 17 (15) | 12 (14) | 5 (16) |
| Not applicable | 472 (60) | 58 (52) | 46 (54) | 16 (50) |
| Phenotype, n (%) | ||||
| B-cell | 523 (67) | 70 (63) | 52 (61) | 20 (63) |
| T-cell | 261 (33) | 42 (37) | 33 (39) | 12 (38) |
| CNS involvement, n (%) | 55 (7) | 5 (5) | 3 (4) | 2 (6) |
| WBC count at diagnosis, median (IQR), G/l | 12 (4-42) | 13 (4-47) | 13 (4-49) | 14 (3-51) |
| Hemoglobin level at diagnosis, median (IQR), g/dL | 10 (8-12) | 11 (9-13) | 10 (8-12) | 12 (10-14) |
| Platelet count at diagnosis, median (IQR), G/l | 72 (32-154) | 89 (40-196) | 94 (32-207) | 84 (56-167) |
| Prothrombin ratio at diagnosis, median (IQR) | 82 (72-92) | 85 (73-92) | 85 (74-92) | 85 (71-90) |
| Fibrinogen level at diagnosis, median (IQR), g/L | 4 (3-5) | 4 (3-5) | 4 (3-6) | 4 (2-6) |
| Poor early PB blast clearance, n (%) | 184 (24) | 19 (17) | 15 (18) | 4 (13) |
Univariable analysis of risk factors for DVT/PE and CVT
| . | Univariable analysis of risk factors for any thrombosis . | Univariable analysis of risk factors for DVT/PE . | Univariable analysis of risk factors for CVT . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | OR . | 95% CI . | P . | OR . | 95% CI . | P . | OR . | 95% CI . | P . |
| Age | 1.02 | 1.00-1.03 | .03 | 1.03 | 1.00-1.04 | .01 | .99 | .97-1.02 | .57 |
| Female | 1.50 | 1.00-2.23 | .05 | 1.32 | .84-2.08 | .23 | 1.54 | .76-3.13 | .23 |
| BMI | 1.04 | 1.00-1.08 | .05 | 1.06 | 1.02-1.10 | .01 | .97 | .90-1.05 | .43 |
| Smoking | .98 | .92-1.04 | .5 | .73 | .42-1.24 | .24 | 1.10 | .49-2.46 | .82 |
| Contraception | .63 | .40-1.75 | .63 | .93 | .40-2.14 | .86 | .58 | .15-2.26 | .43 |
| B-cell phenotype | 1.24 | .82-1.88 | .31 | 1.31 | .82-2.09 | .25 | 1.21 | .58-2.52 | .61 |
| CNS involvement | .58 | .23-1.49 | .26 | .46 | .14-1.49 | .19 | .88 | .20-3.77 | .86 |
| WBC count at diagnosis, ×109/L | .99 | .99-1.00 | .46 | .99 | .99-1.00 | .14 | 1.00 | .99-1.01 | .34 |
| Hemoglobin level at diagnosis, g/dL | 1.05 | .98-1.12 | .19 | 1.00 | .93-1.06 | .93 | 1.17 | 1.03-1.33 | .01 |
| Platelet count at diagnosis/100 × 109/L | 1.02 | 1.00-1.04 | .03 | 1.02 | 1.00-1.04 | .07 | 1.01 | .98-1.05 | .54 |
| Prothrombin ratio at diagnosis, % | 1.00 | .99-1.02 | .23 | 1.00 | .99-1.03 | .15 | .99 | .98-1.02 | .95 |
| Fibrinogen level at diagnosis, g/L | .99 | .98-1.01 | .77 | .99 | .98-1.01 | .80 | .97 | .80-1.19 | .80 |
| Overt DIC | .31 | .08-1.32 | .11 | .44 | .10-1.86 | .26 | — | — | .99 |
| Poor early PB blast clearance, % | .63 | .37-1.07 | .09 | .68 | .38-1.22 | .20 | .45 | .16-1.31 | .14 |
| . | Univariable analysis of risk factors for any thrombosis . | Univariable analysis of risk factors for DVT/PE . | Univariable analysis of risk factors for CVT . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | OR . | 95% CI . | P . | OR . | 95% CI . | P . | OR . | 95% CI . | P . |
| Age | 1.02 | 1.00-1.03 | .03 | 1.03 | 1.00-1.04 | .01 | .99 | .97-1.02 | .57 |
| Female | 1.50 | 1.00-2.23 | .05 | 1.32 | .84-2.08 | .23 | 1.54 | .76-3.13 | .23 |
| BMI | 1.04 | 1.00-1.08 | .05 | 1.06 | 1.02-1.10 | .01 | .97 | .90-1.05 | .43 |
| Smoking | .98 | .92-1.04 | .5 | .73 | .42-1.24 | .24 | 1.10 | .49-2.46 | .82 |
| Contraception | .63 | .40-1.75 | .63 | .93 | .40-2.14 | .86 | .58 | .15-2.26 | .43 |
| B-cell phenotype | 1.24 | .82-1.88 | .31 | 1.31 | .82-2.09 | .25 | 1.21 | .58-2.52 | .61 |
| CNS involvement | .58 | .23-1.49 | .26 | .46 | .14-1.49 | .19 | .88 | .20-3.77 | .86 |
| WBC count at diagnosis, ×109/L | .99 | .99-1.00 | .46 | .99 | .99-1.00 | .14 | 1.00 | .99-1.01 | .34 |
| Hemoglobin level at diagnosis, g/dL | 1.05 | .98-1.12 | .19 | 1.00 | .93-1.06 | .93 | 1.17 | 1.03-1.33 | .01 |
| Platelet count at diagnosis/100 × 109/L | 1.02 | 1.00-1.04 | .03 | 1.02 | 1.00-1.04 | .07 | 1.01 | .98-1.05 | .54 |
| Prothrombin ratio at diagnosis, % | 1.00 | .99-1.02 | .23 | 1.00 | .99-1.03 | .15 | .99 | .98-1.02 | .95 |
| Fibrinogen level at diagnosis, g/L | .99 | .98-1.01 | .77 | .99 | .98-1.01 | .80 | .97 | .80-1.19 | .80 |
| Overt DIC | .31 | .08-1.32 | .11 | .44 | .10-1.86 | .26 | — | — | .99 |
| Poor early PB blast clearance, % | .63 | .37-1.07 | .09 | .68 | .38-1.22 | .20 | .45 | .16-1.31 | .14 |
Platelet count at diagnosis is reported as the platelet count divided by 100.
DIC, diffuse intravascular coagulation.
Poor prognostic factors, such as ALL phenotype, high WBC (>30 × 109/L), CNS involvement, and poor prednisone response, were not associated with an increased risk of thrombosis. Hereditary risk factors for thrombosis (protein S deficiency, protein C deficiency, activated protein C, prothrombin G20210A, and factor V Leiden polymorphisms) and lupus anticoagulant were seldom evaluated (42 of 110 patients [38%] with thrombosis and 191 of 677 [28%] without thrombosis were tested), and their impact on thrombosis could not be evaluated. Prior VTE was not associated with an increased risk of thrombosis during treatment. Oral contraceptives before diagnosis and a history of smoking were not associated with thrombosis. There was no association between rituximab or cyclophosphamide randomization and thrombotic events (P = .57 and P = .12, respectively).
The impact of thrombosis prophylaxis during induction therapy
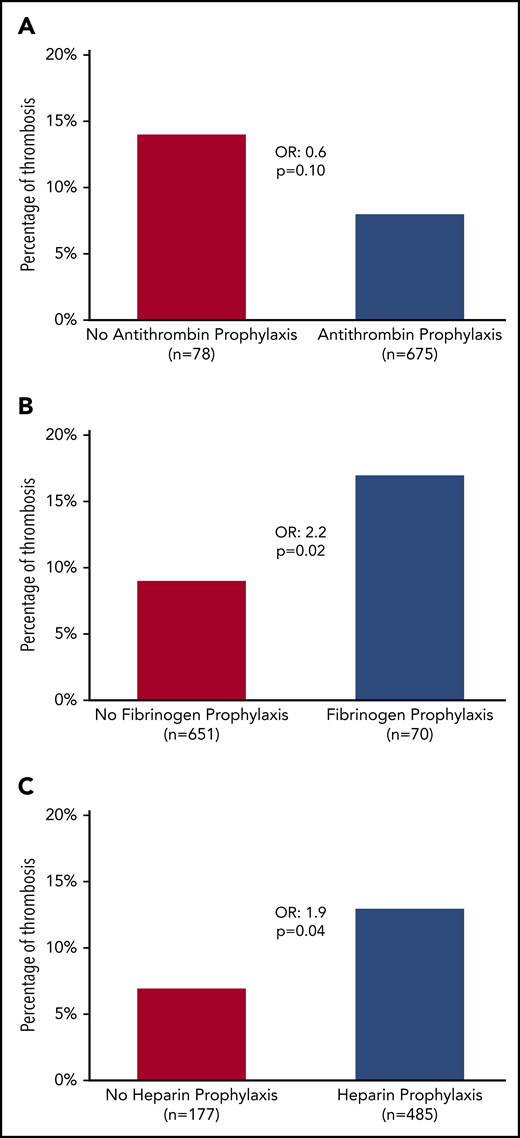
During induction, VTE occurred after a median of 3 L-ASP infusions (median time: day 19, from day −8 to day 49) with no difference between DVT/PE and CVT (median, day 18 for DVT/PE vs day 19 for CVT; P = .6). The median AT nadir was 51% (IQR, 45%-58%), with no difference between patients with and without thrombosis (Table 3). Prophylactic AT concentrates were administered in 64 (82%) and 620 (88%) patients, with and without thrombosis, respectively (P = .05; Table 3). After we excluded patients who did not receive L-ASP (n = 8), who developed thrombosis before L-ASP (n = 8), or both (n = 2), we found that AT supplementation did not have a significant impact on VTE (8% vs 14%; OR, 0.6; 95% CI, 0.28-1.1; P = .1; Figure 2A).
Prophylactic measures in patients without and with VTE during induction
| . | All patients (n = 784) . | No thrombosis during induction (n = 706) . | Thrombosis during induction (n = 78) . | P . |
|---|---|---|---|---|
| L-ASP injections, n (IQR) | 8 (5-8) | 8 (5-8) | 6 (3-8) | <.001 |
| AT nadir, n (IQR) | 51 (45-58) | 51 (45-58) | 51 (44-62) | .66 |
| AT prophylaxis, n (%) | 684 (87) | 620 (88) | 64 (82) | .05 |
| FFP prophylaxis, n (%) | 76 (10) | 68 (10) | 8 (10) | .82 |
| Fibrinogen prophylaxis, n (%) | 70 (9) | 58 (8) | 12 (15) | .02 |
| Heparin prophylaxis, n (%) | ||||
| No | 177 (23) | 164 (23) | 13 (17) | .04 |
| Yes | 485 (62) | 421 (60) | 64 (82) | — |
| Missing data | 122 (15) | 121 (17) | 1 (1) | — |
| . | All patients (n = 784) . | No thrombosis during induction (n = 706) . | Thrombosis during induction (n = 78) . | P . |
|---|---|---|---|---|
| L-ASP injections, n (IQR) | 8 (5-8) | 8 (5-8) | 6 (3-8) | <.001 |
| AT nadir, n (IQR) | 51 (45-58) | 51 (45-58) | 51 (44-62) | .66 |
| AT prophylaxis, n (%) | 684 (87) | 620 (88) | 64 (82) | .05 |
| FFP prophylaxis, n (%) | 76 (10) | 68 (10) | 8 (10) | .82 |
| Fibrinogen prophylaxis, n (%) | 70 (9) | 58 (8) | 12 (15) | .02 |
| Heparin prophylaxis, n (%) | ||||
| No | 177 (23) | 164 (23) | 13 (17) | .04 |
| Yes | 485 (62) | 421 (60) | 64 (82) | — |
| Missing data | 122 (15) | 121 (17) | 1 (1) | — |
Incidence of thrombosis according to prophylactic measures. (A) AT. (B) Fibrinogen. (C) Heparin.
Incidence of thrombosis according to prophylactic measures. (A) AT. (B) Fibrinogen. (C) Heparin.
Fibrinogen concentrates were administered to 70 patients (9%) with hypofibrinogenemia, even though none of the patients had a grade 3 to 4 bleeding event (Table 3). Patients who received fibrinogen concentrates experienced VTE more often (17% vs 9%; OR, 2.2; 95% CI, 1.1-4.3; P = .02; Figure 2B). The prophylactic administration of FFP had no impact on VTE. Heparin was prophylactically administered in 485 patients (62%). Surprisingly, most patients (82%) who subsequently experienced VTE received heparin prophylaxis (13% vs 7% for those who did not receive heparin prophylaxis; OR, 1.9; 95% CI, 1-3.6; P = .04; Table 3; Figure 2C).
Although patients who received heparin prophylaxis also had a higher platelet count at diagnosis of ALL in comparison with those who did not (79 vs 59 × 109/L; P = .05), the association between heparin prophylaxis and an increased risk of VTE persisted after adjustment for platelet counts at diagnosis (supplemental Table 1). Specifically, there was no difference in the incidence of personal and familial thromboembolic events in patients who did or did not receive heparin prophylaxis. Patients who received heparin prophylaxis during induction had similar platelet counts during induction therapy with a similar number of patients with platelets <20 × 109/L at any time (P = .14) and a similar number of days spent with platelets below that level (P = .17), and thus were not recommended for additional platelet transfusion. Patients who experienced grade 3 to 4 bleeding complications were less likely to receive heparin prophylaxis, but the result did not reach statistical significance (59% vs 74% of patients without bleeding complications; P = .09). Of interest, patients who received heparin prophylaxis with no AT prophylaxis (38 patients; 6%) were more likely to experience thrombosis (24%) in comparison with other patients (603 patients; 94%) who experienced 9.6% of the VTEs (P = .02; supplemental Table 2).
CVT occurred in 29 patients during induction therapy from days 1 to 31 with a median time to occurrence of 19 days from the beginning of induction therapy. All but 2 received IT injections during induction (2 patients received 1 IT, 11 received 2, and 14 received 3). Patients with CVT received fewer L-ASP infusions (median, 3 vs 8 for patients without thrombosis; P < .001), had thus a higher AT nadir (median, 60% vs 51% and 47% for patients without thrombosis and with DVP/PE, respectively; P < .001), and did not require supplementation as often (69% of patients vs 88% and 90% of patients without thrombosis and with DVP/PE, respectively; P = .003). CVT also occurred more often in patients receiving heparin prophylaxis (90% of patients vs 60% and 78% of patients without thrombosis and with DVP/PE, respectively; P = .04; supplemental Table 3).
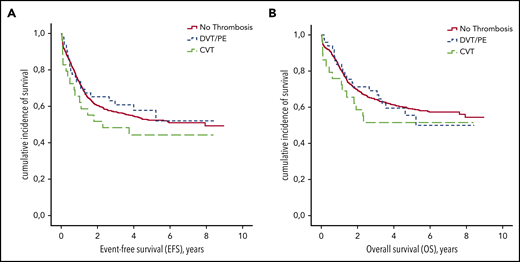
Patients with VTE received fewer L-ASP infusions during induction therapy (median number of 6 vs 8 injections for those without VTE; P < .001; Table 3). The occurrence of DVT/PE or CVT during induction therapy had no impact on EFS or OS (P = .47 and P = .56, respectively; Figure 3). On the other hand, the number of L-ASP injections received during induction was associated with EFS (hazard ratio [HR], 0.8; 95% CI, 0.8-0.9; P < .001) and OS (HR, 0.8; 95% CI, 0.8-0.9; P < .001).
EFS and OS according to thrombosis during induction therapy. (A) EFS. (B) OS.
There was no association between VTE and other grade 3 and 4 toxicities, such as hypertriglyceridemia (P = 0.99), diabetes (P = 0.99), hepatotoxicity (P = .8), and pancreatitis (P = .34) during induction therapy.
Administration of L-ASP during subsequent phases of treatment
Patients who received L-ASP during subsequent consolidations did not have an increased risk of thrombosis in comparison with patients who did not receive L-ASP (P = .61; P = .92; and P = .57 during consolidation courses 1, 2, and 3, respectively). Also, the dose of L-ASP received during these treatment phases was not associated with an increased risk of thrombosis (P = .77; P = .39; and P = .86 during consolidation courses 1, 2, and 3, respectively).
Patients with standard-risk ALL (n = 354) and/or without a donor received late intensification therapy. Patients experiencing VTE before late intensification were less likely to receive L-ASP during this treatment phase (34 and 52 patients [65%] with VTE vs 265 and 302 patients [88%] without VTE; P < .001). Previous VTEs had no impact on the rate of VTEs during late intensification (OR, 1.8; 95% CI, 0.4-8.7; P = .5). Of the 34 patients with VTE before late intensification who were rechallenged with either E coli L-ASP or Erwinase L-ASP during late intensification, only 1 experienced VTE recurrence (3%). During late intensification, fewer patients received AT, FFP, fibrinogen concentrates, and heparin prophylaxis than during induction therapy (58% vs 87%, 2% vs 10%, 5% vs 9%, and 36% vs 62% during induction therapy, respectively). Prophylactic measures had no impact on the incidence of thrombosis during late intensification (supplemental Table 4).
Safety of the prophylactic strategy
Thirty-nine (5%) grade 3 to 4 bleeding complications were reported during the full treatment period, most of them (31 of 39 events, 80%) occurring during induction therapy (Figure 4A). Among them, digestive bleeding was the most frequent (14 of 39; 36%) followed by intracranial bleedings (12 of 39 events; 31%; Figure 4B). Of note, 5 of 12 (42%) of grade 3 and 4 intracranial bleedings were secondary to CVT. Neither patients who received AT concentrates and heparin prophylaxis (OR, 0.7; 95% CI, 0.3-1.8; P = .4; and OR, 0.5; 95% CI, 0.2-1.1; P = .1, respectively), nor patients with VTE experienced more grade 3 and 4 bleedings (6 of 112 vs 34 of 672 bleeding events; OR, 1.1; 95% CI, 0.4-2.6; P = .9).
Timing and type of grade 3 to 4 bleeding complications. (A) Distribution of bleeding events according to treatment phase. (B) Distribution of bleeding type.
Timing and type of grade 3 to 4 bleeding complications. (A) Distribution of bleeding events according to treatment phase. (B) Distribution of bleeding type.
Discussion
This study on thrombotic prophylaxis in adult ALL patients is based on the multicenter prospective GRAALL-2005 study. Although it was not powered to demonstrate the efficacy and safety of the prophylactic strategy, it describes the potential hazards of fibrinogen supplementation, whereas FFP and heparin seemed ineffective. Despite extensive AT supplementation, many patients experienced VTE. This prophylactic strategy was not associated with an increased hemorrhagic risk. In this study, the type of VTE (DVT/PE and CVT) did not have the same risk factors.
As shown by others, the rate of DVT was increased in our adult cohort in comparison with groups in pediatric studies that carried similar rates of CVT.14-16,29-32 We found a near threefold increase in VTE in our study in comparison with previous data compiled from small retrospective studies. The prospective nature of our study enables the exhaustive identification of most VTEs. VTE may therefore be underrecognized in previous studies. In the literature, the risk of DVT/PE is associated with age, comorbidities, obesity, and placement of a central venous line (CVL).30,33,34 This last parameter could not be evaluated in our study because all patients had CVLs. Increased use of CVLs, which is recommended for all patients in our protocol, may have caused the higher thrombotic rate in our study as we observed 50 DVT of the upper limb, for which CVL is a recognized risk factor.30 In contrast to DVT/PE, the only risk factor for CVT was hemoglobin levels at diagnosis. In the literature and in our study, most CVT occurred during intensive L-ASP treatment with fewer events occurring later on (3 of 32 events during subsequent phases).35-37
To date, AT supplementation is the most effective reported prophylactic measure to prevent thrombosis in this setting.22,23 In most clinical studies, AT supplementation was associated with a decreased incidence of thrombosis but randomized trials are lacking.18,24,25,38-40 In our study, despite extensive supplementation (87%), many patients experienced VTE, which did not completely prevent this risk. The lack of statistical power because of the low number of patients not receiving AT supplementation in our cohort may explain the lack of statistical difference. Moreover, this prophylactic strategy was safe and was not associated with an increased risk of severe bleeding. A substantial proportion of patients receiving AT supplementation still had AT levels lower than the cutoff target of 60%. A more rigorous supplementation may be of benefit to patients in preventing VTE. The threshold levels for AT supplementation should be evaluated prospectively.41 The benefit of using a higher threshold (>60%) for administrating AT should be evaluated in this context.25 A recent randomized trial showed that pediatric patients with an AT activity target of 80% experienced a lower incidence of VTE than those who received unfractionated heparin (1.9% vs 8%; P < .001).40 A systematic AT supplementation with high AT doses once a week or every day during L-ASP infusions has also been proposed.38,42 The cost effectiveness of this procedure remains to be determined. This systematic AT replacement strategy could be explored in patients at high risk of thrombosis risk (older age, higher BMI) and/or in those with higher circulating tissue factor which is found to be a highly relevant predictor factor for recurrent VTE in cancer patients.43
The administration of fibrinogen concentrates in case of hypofibrinogenemia has seldom been reported in the literature.18,44,45 Herein, fibrinogen concentrates effectively increased fibrinogen levels but were associated with an increased risk for thrombosis (OR, 2.2; 95% CI, 1.1-4.3; P = .02). Fibrinogen concentrates should be restricted to severe but rare bleeding associated with hypofibrinogenemia. Prophylactic supplementation with FFP had no impact on VTE. In this setting, it has been previously evaluated in 2 studies including 240 and 719 patients, respectively, with only a moderate impact in 1 of them (6% of VTE in those who received FFP vs 19% in those who did not), whereas the other study did not show any difference.17,20 In any case, a hypothetical role on the prevention of thrombosis remains to be explained, because it does not modify plasmatic AT levels.18,19,46 Moreover, using FFP may lower the activity of L-ASP on lymphoblasts related to exogenous asparagine repletion.21
The impact of heparin prophylaxis on reducing the incidence of thrombosis in ALL patients with L-ASP–containing regimens is debated.40,47,48 In our study, even though all patients were supposed to receive heparin prophylaxis, all did not receive such therapy. We observe that it was more frequently administered to patients with less intense thrombocytopenia. After adjustment for platelet counts at diagnosis, heparin prophylaxis was surprisingly associated with an increased risk of VTE. We have no clear explanation for this observation, and the lack of details regarding the modality of heparin prophylaxis in our study (type, dose, and therapeutic level monitoring) limits the generalization of this result. Patients deemed to be at increased bleeding risk and thus at decreased risk of thrombosis, may have received less heparin prophylaxis. The extensive use of unfractionated heparin in our study may also explain the lack of efficacy of heparin prophylaxis that we observed. Given the severe AT depletion induced by asparaginase, unfractionated heparin is probably not the most effective anticoagulant in this setting, whereas some data support the use of LMWH (3.5% of VTE vs 8% in those who received unfractionated heparin).40 The superior efficacy of LMWH in comparison with unfractionated heparin should be evaluated prospectively in adult patients. Direct anticoagulants have yet to be evaluated in clinical studies, especially regarding bleeding complications, because they may interact with other medications.49 A randomized trial comparing apixaban to standard of care for prevention of thrombotic events in children is currently enrolling pediatric patients (PREVAPIX-ALL).
VTEs were less frequently observed in subsequent phases of treatment. During late intensification, the incidence of VTE was only 2.5%, despite the use of an identical schedule of L-ASP protocol as during induction phase and the lower rate of prophylactic measures administered to the patients. This seems to indicate that thrombosis during ALL is related, not only to treatment with L-ASP, but also to the disease itself, as concluded by others.9,10 It is also possible that silent inactivation of L-ASP during late intensification decreased the rate of VTE.50,51 The potential association between asparaginase activity and risk of thrombosis will be studied in GRAALL-2014, currently in the enrolling phase, where these levels are prospectively recorded. Because of this lower risk of thrombosis during late intensification, L-ASP has been reintroduced safely, especially among 34 patients who experienced VTE before late intensification in which only 1 recurrence of VTE was observed after L-ASP rechallenge.16 Reexposure to L-ASP appears therefore to be safe in patients who experience VTE, provided they receive anticoagulation therapy.52
Of note, E coli L-ASP used in this study is no longer available in the United States, and pegylated L-ASP is more extensively used in current pediatrics-inspired protocols. The latter formulation of L-ASP will probably be used in the future GRAALL-2020 protocol. Thromboembolism remains a major cause of toxicity in adult patients receiving pegylated L-ASP, with 5% to 18% of patients experiencing such a complication.32,53-59 The rate of thrombotic complications appears similar in cohorts comparing native and pegylated L-ASP.58,60 Even though lowering pegylated L-ASP doses reduces global toxicity with a similar efficacy, the rate of thromboembolic complications is unchanged.55,61 The impact of prophylactic AT supplementation remains to be determined in this setting.53 It is of the utmost importance to evaluate prophylactic measures against thromboembolic complications in this setting.
In summary, in ALL patients receiving L-ASP therapy, extensive AT prophylaxis did not completely prevent the risk of VTE. Maintaining higher AT levels in patients at increased risk for VTE should be evaluated. Fibrinogen concentrates increase the risk of thrombosis and should be restricted to patients with hemorrhage. The impact of heparin prophylaxis must be reevaluated, as it was associated with an increased risk of VTE in our study. L-ASP can be safely reintroduced in patients who experienced VTE during induction. This large descriptive study could be the basis for future randomized studies evaluating new antithrombotic prevention measures in the GRAALL protocols.
For original data, please e-mail the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the Regional Clinical Research Office, Paris, and by grants from the Programme Hospitalier de Recherche Clinique (PHRC), French Ministry of Health, and Institut National du Cancer in France (PHRC AOM 04144–P040429), and the Swiss State Secretariat for Education, Research, and Innovation (SERI; Bern, Switzerland).
Authorship
Contribution: P.C., F.H., N.I., H.D., N.B., M.H.-B. were involved in the conception and design of the study; C.O., N.B., and M.H.-B. analyzed and interpreted the data; and all authors were involved in the collection and assembly of data, in the writing the manuscript, and in the final approval of the manuscript.
Conflict-of-interest disclosure: M.H.-B. had a consultant or advisory role for Erytech. C.O. received honoraria from Incyte and Novartis and M.H.-B from Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
The members of the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) are listed in the supplemental appendix.
Correspondence: Mathilde Hunault-Berger, CHU d’Angers, 4 Rue Larrey, 49933 Angers Cedex 9, France; e-mail: mahunault@chu-angers.fr.
REFERENCES
Author notes
N.B. and M.H.-B. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal