In this issue of Blood, Berentsen and coworkers describe the clinical features of the largest data set (N = 232) of patients with confirmed cold agglutinin disease (CAD) from 24 centers in 5 countries, suggesting a climate dependence for prevalence and incidence.1 Furthermore, the long-term follow-up of outcomes of rituximab-based chemotherapy regimens, especially in combination with bendamustine, reveals greater and longer-lasting responses that became deeper over time.
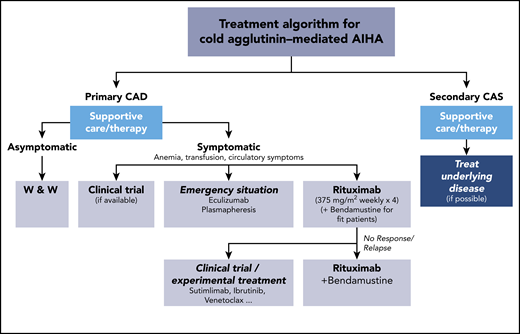
Current treament options for cold agglutinin–mediated AIHA. AIHA, autoimmune hemolytic anemia; W & W, watch and wait.
Current treament options for cold agglutinin–mediated AIHA. AIHA, autoimmune hemolytic anemia; W & W, watch and wait.
In 1952, Schubothe coined the term “cold agglutinin disease” to distinguish the disorder from cold agglutinin syndrome (CAS), which was secondary to an underlying infection or malignancy.2 CAD is a rare form of hemolytic anemia and is challenging in every aspect. The term CAD is quite misleading, because it primarily refers to the physiological properties of the autoantibodies to preferentially bind at cold temperatures, not the clinical presentation of patients as a result of cold temperatures. However, it is characterized by various degrees of chronic hemolysis and even hemolytic crisis, which may be triggered by cold temperatures, as well as by febrile illnesses.3
The underlying pathophysiology is well characterized: CAD is a distinct B-cell lymphoproliferative disorder of the bone marrow leading to the production of (clonal) cold agglutinins, typically an immunoglobulin M autoantibody against red blood cell antigens. These antibodies bind to the red cell surface at low temperature, depending on the thermal amplitude, followed by erythrocyte agglutination and activation of the classical complement cascade. This leads to deposition of C3b on affected erythrocytes and initiation of the terminal complement cascade. C3b-coated erythrocytes are phagocytosed by macrophages of the reticuloendothelial system, primarily in the liver.3
Berentsen and coworkers report, in their impressive cohort of 232 patients, that mean age at diagnosis was 68 years, with a male/female ratio of 0.56. The mean follow-up time was 8 years from diagnosis (range, 0-32 years). Not unexpectedly, 51.7% had cold-induced symptoms at or before diagnosis. Median hemoglobin (Hb) level was 9.2 g/dL, and 26.7% presented with Hb < 8.0 g/dL. Five-year survival was estimated to be 83%, which was significantly higher than reported previously (61%).4 A total of 38 primarily arterial thrombotic events (strokes and myocardial infarction that had no association with the severity of anemia) were experienced by 12.9% of patients. Interestingly, Berentsen et al are the first to describe a fourfold greater prevalence (20.5 vs 5.0 cases/million inhabitants) and incidence (1.9 vs 0.48 cases/million inhabitants/y) in Norway compared with Lombardy (Italy), most likely as a result of lower temperatures provoking more symptoms of CAD.
The next challenge in CAD is treatment, when necessary, in addition to general management considerations, like thermal protection. Unlike warm antibody hemolytic anemia, corticosteroids or other immunosuppressants are not active and, therefore, are not recommended. B-cell–directed therapies with rituximab monotherapy or the combination of rituximab with other drugs, such as bendamustine, have become the standard treatment for symptomatic patients with CAD (see figure).3,5 However, those therapies have obvious limitations, because ≥25% of the patients will not respond. There are also the risk of significant toxicities, potential contraindications, and the length of time for response, which is often weeks or even months.3 Berentsen and coworkers are able to show, at the latest follow-up of 45 patients with bendamustine-rituximab treatment, that there was a further improvement in response increasing from 71% to 78%, with complete responses increasing from 40% to 53% and a response duration > 88 months.1,6 This is encouraging and justifies following patients closely after combination chemotherapy and before starting additional, and potentially unnecessary, treatment.
Despite the response to treatment with an increase or even normalization of Hb, patients often continue to hemolyze, as demonstrated by elevated bilirubin and reticulocyte counts with ongoing symptoms of fatigue or other related complications, as a result of complement activation. Unfortunately, patient-related outcomes were not analyzed in this study.
Taken together, these data show the medical need for new, fast-acting, and safe treatments. As shown recently for the anti-C1s antibody sutimlimab in CAD, this complement-directed therapy is able to shut down hemolytic activity almost immediately.7,8 Therefore, treatment strategies in the future must focus on the foremost problems in CAD (eg, hemolysis, anemia, and acrocyanosis) with the use of suitable regimens and/or combinations of complement-inhibition and B-cell–directed therapies, as reported previously.3,8
Once again, this work by Berentsen et al demonstrates the importance of collecting and analyzing data and information from patients with rare or ultrarare diseases in registries. A prospective global patient registry (CADENCE) for patients with CAD was just launched to further advance the understanding of the clinical presentation, natural history, and effective treatment of this unique disease.9
Conflict-of-interest disclosure: A.R. has received research support from Roche and has received honoraria and acted as a consultant for Alexion Pharmaceuticals; Apellis Pharmaceuticals; Bioverativ, a Sanofi company; Novartis; Roche; and Sanofi.