Background
Lenalidomide, bortezomib, and dexamethasone (RVD) is a standard front-line regimen for both transplant eligible and ineligible multiple myeloma (MM) patients. Based on original APEX study data, which reported development of thrombocytopenia, anemia, and neutropenia in 35%, 26%, and 19%, respectively, complete blood counts before every administration of bortezomib has been recommended. Although an injection of bortezomib takes only 3-5 seconds, waiting for the results of a complete blood count (CBC), with or without a comprehensive metabolic panel (CMP), can add approximately an hour to the visit. This can be cumbersome for patients, who are usually older adults, and it can also present a financial burden for patients, payers, and institutions.
Methods
The primary objective of this study was to identify patients who can receive RVD safely without repeat labs prior to each bortezomib injection. Secondary objectives included evaluation of costs and appointment visit times. Eighty-nine patients were identified in an institutional review board- approved descriptive, retrospective, medical chart review of patients with MM aged ≥ 18 years who received ≥3 cycles of RVD treatment from January 1, 2016 to January 31, 2020. Sixty-eight patients (76%) received bortezomib on a weekly schedule and twenty-one patients (24%) received bortezomib on a twice weekly schedule. Seventy-four patients (83%) were newly diagnosed and fifteen patients were relapsed/refractory when they began treatment with RVD.
Results
Thrombocytopenia
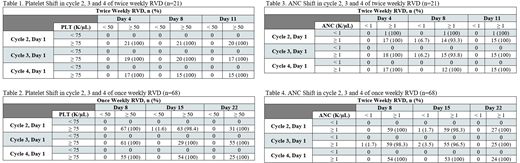
On day 1 of each cycle (starting cycle 2), 100% of patients had a platelet (PLT) count ≥75,000 cells/µL (Table 1 and 2). Less than 10% of patients developed grade ≥ 2 thrombocytopenia, including 2% and 0% with grade 3 and 4 events, respectively. No patients had treatment delays or interventions for any grade of thrombocytopenia. Only one patient (1.6%) had platelets less than 50,000 cells/µL on day 15 of cycle 2. In cycles 3 and 4, 100% of patients had platelets greater than 50,000 cells/µL on each treatment day.
Neutropenia
The majority of patients had an absolute neutrophil count (ANC) ≥ 1,000 cells/µL at the beginning of each cycle: 94%, 100%, and 100% in cycles 2-4, respectively. Of the patients with absolute neutrophils ≥ 1,000 cells/µL on the first day of cycles 2-4, more than 93% of the patients had absolute neutrophils ≥ 1,000 cells/µL on subsequent days of cycles 2-4 (Table 3 and 4).
Grade 3 and 4 neutropenia was observed in 6.7% and 1.1% of patients, respectively. Only two patients (2.9%) had treatment delays. Filgrastim was administered for 3 incidences of grade 3 neutropenia and 1 incidence of grade 4 neutropenia. One patient (1.4%) had a dose reduction in lenalidomide due to grade 3 neutropenia. We did not observe any significant impact of either once a week or twice a week regimens on changes in platelet or absolute neutrophil counts.
Serum Creatinine and Total Bilirubin
No significant trends occurred in serum creatinine or total bilirubin. The average serum creatinine and total bilirubin for both cohorts was ≤ 1.2 mg/dL throughout the study for cycles 1-4.
Wait Time and Costs Implications
We evaluated the time saved and expense reduction by checking labs only on day 1 of each cycle and identified an average time saving of 3 to 4.5 hours and financial savings of $1,542 per cycle for each patient.
Conclusion
This study demonstrates that beyond the first cycle, patients with platelets ≥ 75,000 cells/µL and absolute neutrophils ≥ 1,000 cells/µL on the first day of a cycle do not need labs prior to each administration of bortezomib (while on RVD regimen). Furthermore, a CMP does not need to be obtained prior to each dose of bortezomib, unless otherwise indicated, since no significant trends occurred in serum creatinine or total bilirubin in our study. Decreased monitoring of CBCs and reducing the number of labs needed during each cycle will improve resource utilization, save money, and save time for patients and health care providers. These cytopenias were typically cyclic and transient. Prescribing information for bortezomib and lenalidomide recommend monitoring compete blood counts and platelets at several intervals and neither medication package inserts give guidance on how frequent serum creatinine and total bilirubin should be monitored. With so many recommendations for monitoring, the frequency of obtaining labs is often left at the discretion of the provider.
Munshi:BMS: Consultancy; OncoPep: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; C4: Current equity holder in private company; Janssen: Consultancy; Adaptive: Consultancy; Legend: Consultancy; Amgen: Consultancy; AbbVie: Consultancy; Karyopharm: Consultancy; Takeda: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal