Introduction:
Secondary Central Nervous System Lymphoma (SCNSL) is the spread of a lymphoma to the CNS, the primary focus of which is situated elsewhere in the body. Most commonly, it is a non-Hodgkin lymphoma which either presents as a CNS involvement due to systemic relapse or progression, or as an isolated relapse in the CNS despite systemic remission. Traditionally, it has been treated by intrathecal or systemic chemotherapy and/or WBRT (Whole-brain radiation therapy) but recently, the use of high-dose chemotherapy (HDT) with autologous stem cell transplantation (ASCT) has shown encouraging results. We present a systematic review of literature displaying the treatment outcomes of HDT/ASCT in SCNSL.
Methods:
We performed a comprehensive literature search (following PRISMA guidelines) on July 08 2020 on PubMed, Cochrane Library and Clinicaltrials.Gov by using the relevant MeSH terms of high-dose chemotherapy, autologous stem cell transplantation, CNS lymphoma, efficacy and safety. Following screening by 2 reviewers, we selected 12 published studies (n=353) and included data from these studies in our systematic review. We manually extracted data summarized the results.
Results:
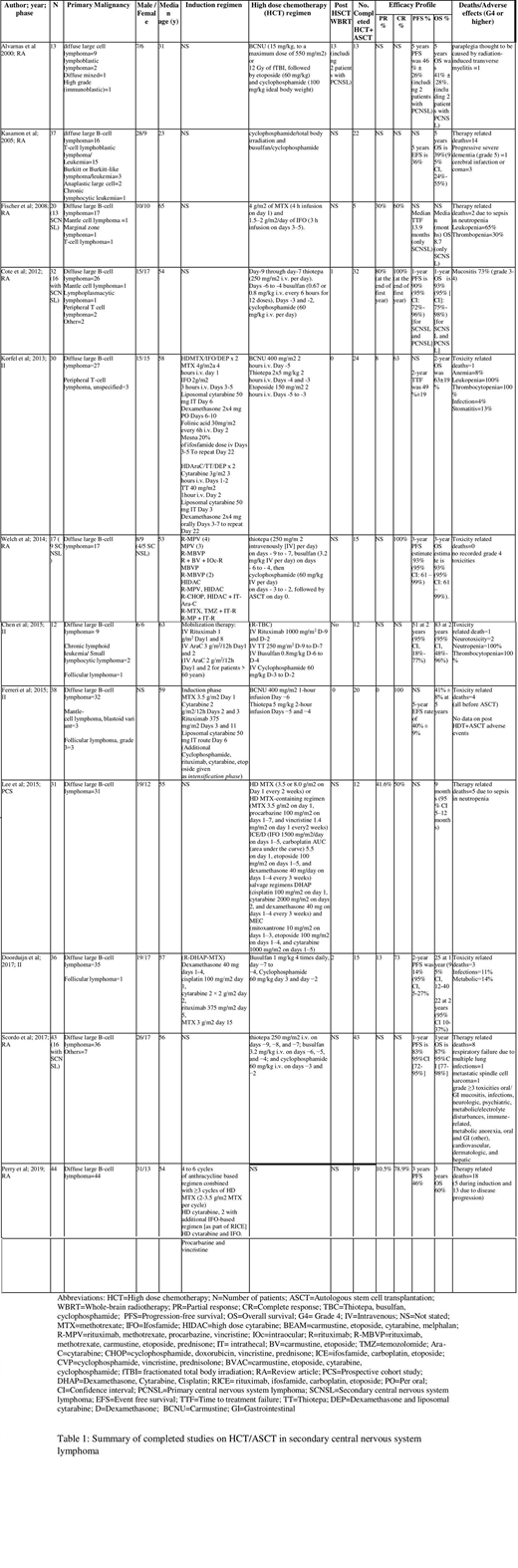
232/353 patients eventually underwent sequential HDT+ASCT who were evaluable for treatment response out of which 190 patients had SCNSL (see table 1).
Carmustine (BCNU) based HDT:
BCNU based regimens have been the most extensively studied HDT among SCNSL patients (n=81). Korfel et al. in a phase 2 trial (2013, n=30) used HDT combination of BCNU, thiotepa and etoposide followed by ASCT in SCNSL patients. No patient was given whole brain radiation therapy (WBRT). The results yielded a PR of 8%, CR of 63% and 2-year OS of 63%. 1 toxicity related mortality (TRM) was noted and >grade 4 adverse effects included infection (4%) and stomatitis (13%). Ferreri et al. (2015, n=38) in a phase 2 trial use HDT with BCNU plus thiotepa on 38 patients 20 of whom underwent subsequent ASCT. The results showed CR of 100%, event free survival (EFS) of 40% ±9% and OS of 41% ±8% at 5 years with four patients suffering TRM.
Thiotepa, busulfan and cyclophosphamide (TBC) based HDT:
The combination based on TBC has been a popular option for SCNSL patients (n=53). In a retrospective analysis by Welch et al. (2014, n=17), TBC combination followed by sequential ASCT was studied in both primary and SCNSL patients (primary malignancy being diffuse large B-cell lymphoma) and the collective results showed complete response (CR) of 100%, PFS of 93% (95% CI: 61-99%) and OS of 93% (95% CI: 61-99%) at 3 years. In addition to the excellent efficacy response, no TRM or grade 4 toxicities were documented. Chen et al. (2015, n=12) in a phase 2 trial used HDT with rituximab and TBC (R-TBC) combination followed by ASCT rescue in 12 SCNSL patients. At 2 years, the progression free survival (PFS) was 51% (95% CI: 18-77%) and overall survival (OS) was 83% (95% CI: 48-96%). Two patients developed neurotoxicity and one TRM was recorded.
Methotrexate (MTX) based HDT:
MTX based combinations have also been widely used as HDT preceding ASCT in SCNSL patients (n=51). Fischer et al. (2008, n=20) studied HDT of MTX plus ifosfamide (IFO) in a retrospective analysis which showed PR of 30%, CR of 60% and median OS of 8.7 months and two TRM (due to sepsis in neutropenia) in SCNSL patients. Lee et al. (2015, n=31) in a prospective cohort study evaluated HDT with MTX based multidrug combination with ASCT in SCNSL patients which yielded a PR of 41.6%, CR of 50% and OS of 9 months (95% CI: 5-12 months). Five patients had suffered TRM.
Conclusion:
The combination of HDT with ASCT has yielded promising treatment outcomes both in terms of favorable efficacy rates and reduced rates of toxicity in SCNSL patients proving to be a comparable alternative to traditionally used chemo-radiation. However, the data at present is limited to few phase 2 trials with some being displayed collectively with data of primary CNS lymphoma patients. There is an increased need to carry out randomized phase 3 trials exclusively on SCNSL patients to better predict the efficacy and safety profile of HDT/ASCT in these patients.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal