Introduction: It is anticipated that many patients with hemophilia are likely to switch from one coagulation factor product to a newer agent within the next few years. New replacement factor formulations have been developed using several approaches that have theoretical benefit. However, except for pharmacokinetic properties, it may be difficult to determine whether they are superior to current clinically successful preparations. Rurioctocog alfa pegol (Adynovate®), a pegylated, extended half-life factor VIII (FVIII) concentrate, received United States Food and Drug Administration approval in 2015. Its efficacy and safety for the treatment of hemophilia A have been demonstrated in multiple studies (Konkle et al. 2015; Gruppo et al. 2019; Mullins et al. 2017). The ATHN 2: Factor Switching Study provided the opportunity to longitudinally observe previously treated hemophilia patients switching to rurioctocog alfa pegol to identify dosing regimens, patient satisfaction with the change in therapy, impact on health states and productivity.
Methods: The ATHN 2: Factor Switching Study is sponsored by the American Thrombosis and Hemostasis Network (ATHN) and is being conducted at ATHN-affiliated sites in the US. This multi-center, longitudinal, observational study enrolled male and female children and adults with moderate or severe congenital hemophilia A or B (factor VIII or IX clotting activity ≤5% of normal) who were previously treated with plasma-derived or recombinant factor replacement products with ³50 exposure days. Patients receiving care from one of 30 hemophilia treatment centers were enrolled into 2 arms: 1) a prospective arm including those switching factor replacement products who were followed for <1 year; and 2) a retrospective arm including those who had switched factor replacement products within the past 50 weeks at the time of enrollment. Patients were assessed retrospectively or followed prospectively for <1 year. Treatment administered in ATHN 2 was at the discretion of the patient's provider. Each patient was seen during a study visit or contacted by telephone at least once every 3 months. A product-specific module developed for rurioctocog alfa pegol included assessment of dosing and treatment satisfaction vs prior therapy; adherence to therapy assessed using Validated Hemophilia Regimen Treatment Adherence Scale-Prophylaxis (VERITAS-Pro), a validated instrument with 6 subscales (timing, dosing, planning, remembering, skipping, communication); health states measured by the EQ-5D-DL; work productivity assessed by the Work Productivity Activity Impairment Questionnaire (WPAI); and overall satisfaction with therapy.
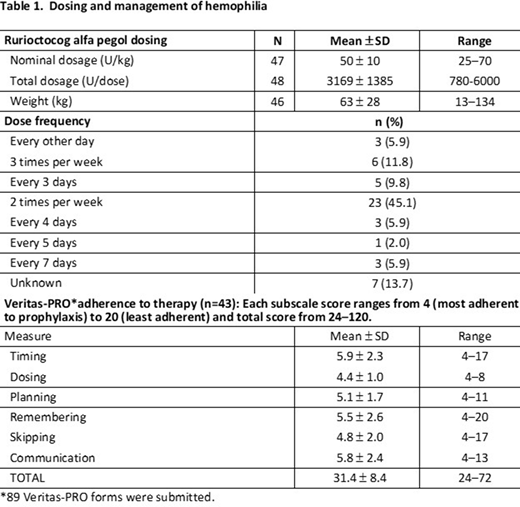
Results: Patients (n=59) with hemophilia A were enrolled into the rurioctocog alfa pegol sub-study by May 1, 2020. Mean (± standard deviation [SD]) age was 25 ± 18 years (range = 4-77 years); 72.9% (n=43) were in the prospective arm and 27.1% (n=16) in the retrospective arm; 11.9% (n=7) had moderate hemophilia A and 88.1% (n=52) had severe disease. All were taking rurioctocog alfa pegol as prophylaxis. At study end, results for dosing (Table 1) indicated the most common treatment regimen was twice weekly (45.1%, n=23), and mean nominal dose was 50 ± 10 U/kg. Patients were highly adherent to prophylaxis with rurioctocog alfa pegol with a mean total VERITAS-Pro score of 31.4 ± 8.4; adherence improved slightly over time (32.6 ± 9.0 at baseline vs 29.2 ± 4.2 at month 12). Patients strongly preferred rurioctocog alfa pegol vs all prior factor products for controlling bleeding, convenience, finding time to take and ease of administering FVIII, and how often they were required to take factor (Table 1). Overall, 76.5% of patients were very satisfied or satisfied with rurioctocog alfa pegol. Assessment of health states indicated that patients generally had no or slight problems with mobility, activities, or self-care; and were not in pain or anxious/depressed. Patients generally missed no time from work or school. Overall health was self-rated at 89.0 ± 13.0 on a scale from 0 (worst health) to 100 (best health) and remained stable from baseline (88.0 ± 14.0) to month 12 (87.8 ± 15.3).
Conclusions: Moderate and severe hemophilia A patients enrolled in ATHN 2 who received rurioctocog alfa pegol for prophylaxis enjoyed excellent health, had little to no school or work impairment and were adherent to, and satisfied with, their treatment regimen.
Recht:Spark: Research Funding; CSL Behring: Consultancy, Other: personal fees; Novo Nordisk: Consultancy, Other: personal fees, Research Funding; Genentech: Consultancy, Other: personal fees, Research Funding; Pfizer: Consultancy, Other: personal fees; BioMarin: Research Funding; Takeda: Consultancy, Other: personal fees, Research Funding; uniQure: Consultancy, Other: personal fees, Research Funding. Guelcher:Novo-Nordisk: Consultancy; Octapharma: Consultancy; Genentech, Octapharma, CSL Behring, Takeda, Pfizer, Novo Nordisk: Consultancy; Takeda: Consultancy. Neufeld:Bayer: Other: DSMB; genetech: Consultancy; Novo Nordsik: Consultancy; Octapharma: Consultancy; Takeda: Consultancy; Imara Pharma: Other: DSMB service; ApoPharma/Chiezi: Other: DSMB service; Pfizer: Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy; Acceleron Pharma: Consultancy, Other: DSMB. Ragni:Alnylam/Sanofi, ATHN, BioMarin, Bioverativ, Sangamo, Spark: Research Funding; Alnylam/Sanofi, BioMarin, Bioverativ, Spark: Consultancy; BioMarin: Consultancy, Research Funding; Bioverativ: Consultancy, Research Funding; Spark: Consultancy, Research Funding; Takeda: Research Funding; Sangamo: Consultancy, Research Funding; Alnylam Pharmaceuticals Inc., Baxalta/Takeda, BioMarin, Bioverativ, and Spark Therapeutics: Membership on an entity's Board of Directors or advisory committees; American Thrombosis Hemostasis Network: Other: Committee work; Baxalta/Takeda, CSL Behring, Genentech, a member of the Roche Group, OPKO Biologics, and Vascular Medicine Institute: Research Funding. Sidonio:Biomarin: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Research Funding, Speakers Bureau; Grifols: Research Funding; Spark: Consultancy, Honoraria; Uniqure: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Novo Nordisk: Consultancy, Honoraria; Octapharma: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria; Genentech: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; Bayer: Consultancy, Honoraria. Takemoto:Novartis: Other: DMBC; Genentech: Consultancy. Tarantino:Spark: Membership on an entity's Board of Directors or advisory committees; CDC: Membership on an entity's Board of Directors or advisory committees; Dova: Membership on an entity's Board of Directors or advisory committees; Biomarin: Membership on an entity's Board of Directors or advisory committees; NovoNordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Research Funding; Octapharma: Membership on an entity's Board of Directors or advisory committees; Grifols: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genentech: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; HRSA: Membership on an entity's Board of Directors or advisory committees; Pfizer: Other; Sobi: Membership on an entity's Board of Directors or advisory committees. Caicedo:Takeda: Current Employment. Denne:Takeda: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal