Introduction: The Khorana Score (KS) is a validated tool to assess the risk of thrombosis in cancer patients, prior to initiation of chemotherapy. The AVERT and CASSINI studies evaluated prophylactic anticoagulation with apixaban or rivaroxaban for patients with KS of 2 or higher. Both studies demonstrated that while patients were taking prophylactic anticoagulant, the Hazard Ratio of cancer associated thrombosis was approximately 0.4. The NCCN and other societies are now recommending oral anticoagulant prophylaxis should be considered for up to 6 months, for patients with an intermediate or high-risk score of ≥2.
Methods: We have developed a medical record tool, that identifies all MSKCC patients prior to initiation of a new chemotherapy regimen, captures the KS parameters, and generates the KS. Once fully implemented, it is anticipated that this tool and appropriate use of prophylactic anticoagulation, will reduce the burden of cancer associated thrombosis. To accurately document a potential reduction in rates of cancer associated thrombosis, we need to establish current baseline rates of cancer associated thrombosis via a contemporary verification of the KS, reflecting potential changes in oncology in the 12 years since the score was first published.
We applied our KS capture tool to a 2-year period (10/17/2017 to 11/29/2019). 13,992 patients were identified with a new chemotherapy order, of whom 13,176 were found to have cancer and had complete parameters for the KS. We tracked all patients for 6 months, to identify possible episodes of Venous Thromboembolism (VTE). VTE episodes were identified by capturing the impressions of all contrast imaging studies, Doppler ultrasounds, and V/Q scans, combined with keyword and phrase computer identification, and manual review. To identify possible VTE events that occurred at an outside emergency department or hospital, we also queried new prescriptions for therapeutic doses of anticoagulants and ICD10 codes. In this analysis we focus on deep vein thrombosis (DVT) and pulmonary embolism (PE).
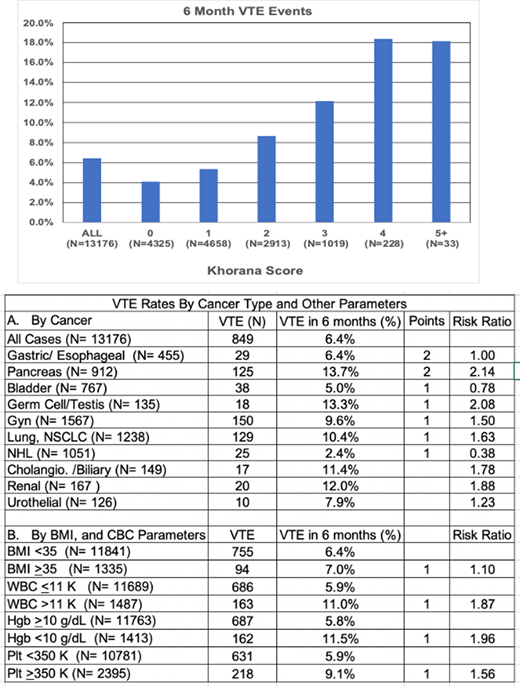
Results: The 6-month rate of VTE (DVT or PE) was 6.4%. The VTE rate was 4.1% in the KS 0 cohort, and >18% for patients with a score of 4 or greater. We assessed the contribution of each parameter of the KS, in univariate analysis. Of the cancers assigned 2 points in the KS, pancreatic cancer had the highest VTE rate of 13.7%, but gastric/esophageal cancer did not have an increased rate. Of the cancers assigned 1 point, germ cell/testis, gynecologic, and lung cancer, also had increased risk of VTE, but bladder and non-Hodgkin lymphoma did not. Renal/urothelial cancers and cholangiocarcinoma/biliary tract cancers were also associated with high rates of VTE, although were not assigned points using the KS. Elevated BMI (≥35 kg/m2) was a weak risk factor. However, baseline anemia (<10 gm/dL), leukocytosis (>11,000/mcL) and thrombocytosis (≥350,000/mcL) were all strong predictors of VTE rate.
Conclusions: The purpose of this QA project was to provide contemporary data on baseline cancer associated thrombosis rates, anticipating implementation of a program of targeted primary thrombosis prophylaxis. It remains clear that the KS provides a valuable risk assessment tool, stratifying 6-month VTE risk from 4.1% to >18%. Cancer type is typically the parameter that gets the first consideration. And most of the cancers with 1 or 2 points did predict increased risk. We don't have an explanation as to why, gastric/esophageal cancers and non-Hodgkin lymphoma were not associated with increased risk, but this finding may reflect the stage of their treatment at which patients begin care at MSKCC. Renal/urothelial, and cholangiocarcinoma/biliary tract cancers are associated with a high risk of VTE, and one may choose to include them for consideration of prophylactic anticoagulation. The relative contribution of pre-chemotherapy blood counts has been questioned in the past, but in this cohort, they clearly contributed to appropriate risk assessment. These data will serve as baseline for future assessment of thrombosis risk reduction upon implementation of a program of targeted prophylactic anticoagulation.
Khorana:Leap: Research Funding; BMS: Honoraria, Research Funding; Merck: Research Funding; Array: Other: Research funding (to institution); Janssen: Honoraria; Bayer: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria; Medscape: Honoraria; Leo Pharma: Honoraria; Seattle Genetics: Honoraria; Pharmacyte: Honoraria; Pharmacyclics: Honoraria. Mantha:MJH Associates: Honoraria; Physicians Education Resource: Honoraria. Soff:Amgen: Research Funding; Amgen: Honoraria; Janssen Scientific Affairs: Honoraria; Dova Pharmaceuticals: Honoraria; Bristol-Myers Squibb, Pfizer: Honoraria; Janssen Scientific Affairs: Research Funding; Dova Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal