Background: Elevated serum ferritin (SF) is common in patients with myelofibrosis (MF) given the frequent red-cell transfusion requirements and impaired iron metabolism. The dysregulation of iron metabolism is associated with increased hepcidin levels which predict for inferior survival in patients with MF (Pardanani et al AJH 2013). High pre-transplant SF negatively impacts overall survival and non-relapse mortality in patients undergoing allogeneic hematopoietic cell transplantation (allo-HCT) for hematological malignancies (Chee et al BMT 2017 & Yan et al 2018). However, the impact of elevated pre-transplant SF specifically on post-alloHCT outcomes has not been described in MF.
Methods: We conducted a retrospective study of patients with MF undergoing allo-HCT at Mayo clinic Rochester, Arizona, and Florida from January 1st 2012 - December 31st 2019. We abstracted demographic and outcome variables to evaluate the impact of pre-transplant ferritin levels on post-transplant outcomes. We used a Receiver Operand Characteristic Curve to identify a pre-transplant serum ferritin (SF) of ≥ 1,123 ng/mL as the cutoff which best correlated with poor survival.
Results: We identified 67 patients with MF who underwent allo-HCT during the study period with readily available data. Patients with a pre-transplant ferritin ≥ 1,123 ng/mL (n=18) had a median age of 59.4 years and were predominantly male (n=9, 50%) with primary myelofibrosis (n=12, 67%), had a DIPSS-plus int-2 (n=10, 56%) and high (n=6, 33%) risk categories and received a reduced-intensity conditioning (n=14, 78%) from a matched-unrelated donor (n=7, 50%) Table1.
Patients with SF ≥ 1,123 ng/mL had a worse overall survival (OS) (median OS= 12.64 months [95%CI=6.08-19.20 months] vs. not-reached (NR) p=0.001). Patients with elevated SF had an increased non-relapse mortality (NRM) rate at 6 months (16.7%, [95%CI=0.4-37.2%]) vs 8.2%,[95%CI= 2.6-18.1%] p=0.021) and 12 months (33.3% [95%CI=13.0-55.4%] vs 10.4% [95%CI=3.8-21.0%] p=0.021)
Patients with ≥ 1,123 ng/mL SF had a non-statistically significant higher rate of acute graft-vs-host disease grades 3-4 (28% vs 12.2% p=0.13) but similar rates of moderate to severe chronic graft-vs-host disease (31% vs 28% p=0.78).
In the univariate analysis, SF ≥1,123 ng/mL and a Hematopoietic stem cell transplant comorbidity index (HCT-CI) ≥3 were associated with an increased risk of mortality (Hazard ratio [HR]=4.18 [95%CI=1.64-10.62] p=0.003] and HR=4.42 [95%CI=1.70-11.46] p=0.003, respectively). Both retained significance in multivariate analysis SF ≥1,123 ng/mL (HR= 2.80 [95%CI= 1.0-7.6], p=0.04)] and HCT-CI score ≥ 3 (HR 3.10 [95%CI= 1.12-8.62, p=0.03]) after adjusting for age, DIPSS-plus score, conditioning regimen intensity, donor type, CMV risk, prior use of ruxolitinib, and ABO mismatch.
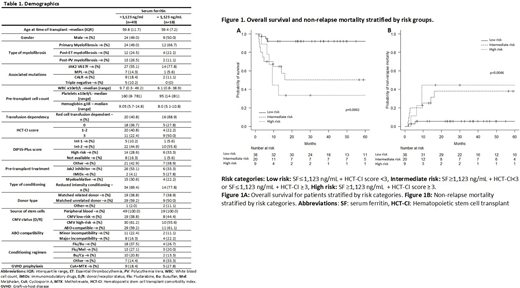
We grouped the patients into low, intermediate and high risk categories using SF level and HCT-CI scores as detailed in Figure 1. The risk category based on SF and HCT-CI was statistically significant in impacting OS and NRM in this cohort. The 12 month OS rate was lower in the high risk category (44.4% [95%CI= 13.6%-71.9%]) when compared to the intermediate (64.6%, [95%CI=39.7%-81.3%]) and low risk (91.9%, [95%CI= 76.9%-97.3%], p=0.0002) categories. Similarly, the 12 month NRM rate was higher in the high risk category (44.4%, [95%CI=11.0%-74.2%]) when compared to the intermediate (25%, [95%CI=8.7%-45.5%]) and low risk (5.3% [95%CI=0.9%-15.9%], p=0.0046) categories.
Conclusion: In patients undergoing allogeneic SCT for myelofibrosis, serum ferritin ≥ 1,123 ng/mL is a predictor of high NRM and inferior OS. Combining ferritin serum levels with HCT-CI may help in better patient selection for allo-HCT in MF population. Larger patient cohort is warranted to confirm our findings.
Kharfan-Dabaja:Daiichi Sankyo: Consultancy; Pharmacyclics: Consultancy. Shah:Dren Bio: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal