INTRODUCTION
Achieving complete remission (CR) is an important goal for patients with acute myeloid leukemia (AML)undergoing treatment with the intent to cure. However, even after consolidation and/or hematopoieticcell transplantation (HCT), many patients will experience disease recurrence. Because of this there has long been an interest in the use of maintenance therapies after the intensive treatment phase to prolong the duration of remission and improve survival and the likelihood of cure. Although the concept of maintenance therapy in AML has been present since the 1960s, it is still a matter of controversy.Azacitidine (AZA) is a hypomethylating agent that acts by incorporating itself into DNA, reversiblyinhibiting DNA methyltransferase, thereby blocking its methylation and activating silenced tumor suppressor genes, resulting in an antitumor effect.Maintenance chemotherapy had not been able to demonstrate improvement in survival. However, in the results of the HOVON-97, Huls et al. showed a 12-month progression free survival (PFS) in 64% of older AML patients who received AZA as maintenance therapy for a maximum of 12 cycles, thus opening the door for new research in this field.
OBJECTIVE
To know if there is an impact of maintenance treatment with 5-Azacitidine on disease-free survival and progression-free survival, in Acute Myeloid Leukemia in complete remission, in patients who are and are not candidates for bone marrow transplant.
PATIENTS AND METHODS
We conducted a longitudinal, cohort, analytical, descriptive and single-center study, in the time period between January 2016 and December 2019 (designated the AZA group). Data obtained from patients receiving maintenance was compared with a historic patient cohort (designates as the no-AZA group).This study was approved by the ethics committee of CMN 20 de Noviembre.
RESULTS
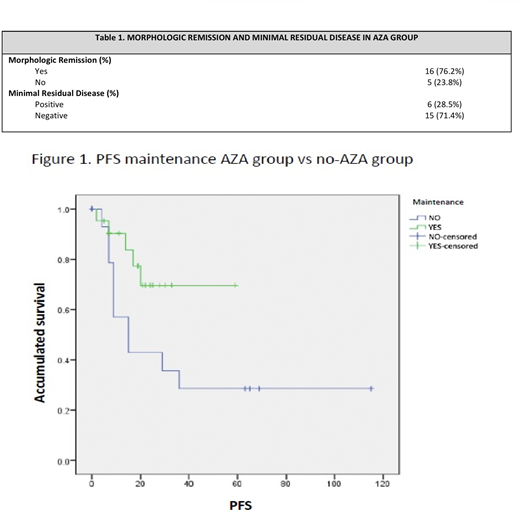
A total of 21 patients were analyzed in the AZA group, of which 53% (n = 12) were women and 37% (n =9) were men, the median age was 54 years with a range of 19 to 65. The median of leukocytes,hemoglobin and platelets of the AZA-group at diagnosis was 22.8x109, 9.3 g/dL, and 44,000 mm3, respectively. Thirty percent had an intermediate cytogenetic risk. In the no-AZA group a total of 18 patients were analyzed, of which 73% (n=11) were female and 26% (n=7) were male, the median age was 57 years with a range of 18 to 64. The median of leukocytes, hemoglobin and platelets at diagnosis was 29.5 x109, 9.5 g/dL, and 57,000 mm3, respectively. In the no-AZA group 8% and 22% had intermediate and high cytogenetic risk, respectively. The median follow up in the no-AZA group was 32 months (10-96). The median of cycles of maintenance with AZA was 12 cycles (1-24); the follow up median, in months, was 19 (range 7-59). The response to maintenance therapy was evaluated after the completion of the first 6 treatment cycles, in which 71.4% maintained a negative minimal residual disease (MRD), and 23.8% experienced a relapse. In the no-AZA group the median time to progression was approximately 8 months, with a PFS median of 15 months; time to progression in the AZA group was approximately 15 months. At the closing of this study the AZA group has not reached the median for PFS (p=0.011) (Figure 1); 42.8% are still in maintenance therapy and in remission, 19% received a bone marrow transplant and are still in remission, 14.4% experienced relapse, 4.8% are in elective suspension of treatment and continue in remission, 9.5% are in observation and palliative care respectively
CONCLUSIONS
Azacitidine used as maintenance treatment in Acute Myeloid Leukemia can extend progression free survival.
De La Peña:Janssen:Speakers Bureau;Novartis:Speakers Bureau;Amgen:Speakers Bureau.Alvarez:Amgen:Speakers Bureau;Roche:Speakers Bureau;Celgene:Speakers Bureau;Novartis:Speakers Bureau;Janssen:Speakers Bureau.Perez:Novartis:Speakers Bureau;Roche:Speakers Bureau;Celgene:Speakers Bureau.Alvarado:Novartis:Speakers Bureau;Amgen:Speakers Bureau;Celgene:Speakers Bureau;Alexion:Speakers Bureau;Roche:Speakers Bureau.
azacitidine as maintenance for AML it is not approved yet in Mexico
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal