Background: Targeted inhibition of the FLT3 tyrosine kinase is a treatment strategy for patients with relapsed or refractory, FLT3 mutation-positive acute myeloid leukemia (R/R FLT3mut+ AML). While the multikinase inhibitors, sorafenib and midostaurin, were not approved specifically for R/R AML, they are used off-label as treatment. Since FDA approval on 11/28/2018, gilteritinib has become the first globally approved targeted therapy for the treatment of R/R FLT3mut+ AML in adults. This study aims to understand the treatment landscape for R/R FLT3mut+ AML patients following the introduction of a new therapeutic agent.
Aim/Objective: To describe the characteristics and treatment patterns of patients initiating FLT3 tyrosine kinase inhibitors (TKIs) for R/R FLT3mut+ AML in the real-world setting.
Methods: This US-based retrospective cohort study was conducted using two separate closed-claims databases (MarketScan, 1/1/2007-2/29/2020; IQVIA, 1/1/2006-4/30/2020) and a specialty-pharmacy claims database (ProMetrics, 12/6/2018-3/12/2020). Patients ≥18 years of age, with an AML diagnosis and newly initiated FLT3-TKI (ie, gilteritinib, midostaurin, or sorafenib) therapy for R/R diagnosis or episode ("R/R AML") on or after gilteritinib market availability were eligible for inclusion. Patients were indexed on the first newly initiated FLT3-TKI for R/R AML observed on or after 12/1/2018. Patients' baseline clinical and demographic characteristics, AML-related treatments preceding FLT3-TKI initiation for R/R AML, and concomitant therapies (defined as any AML-related treatment overlapping with FLT3-TKI use after R/R AML) were identified. Because individual patients could have received more than one FLT3-TKI during the study period, treatment sequencing was analyzed by FLT3-TKI-containing regimens. Given the larger sample size available within the ProMetrics Specialty Pharmacy database, gilteritinib dose patterns and treatment duration were analyzed using de-identified dispensed prescription data from a separate gilteritinib-only cohort for greater precision. Descriptive statistics were used to evaluate all study outcomes, except for treatment duration, which was estimated using Kaplan-Meier life table analysis.
Results: A total of 52 and 51 patients initiating FLT3-TKIs for R/R AML were identified in the MarketScan and IQVIA databases, respectively. The mean age of patients was 53.1 (MarketScan) and 52.3 years (IQVIA); patients had a mean ± SD Quan-Charlson Comorbidity Index of 3.6 ± 2.4 (MarketScan) and 3.7 ± 2.2 (IQVIA). Most patients treated with FLT3-TKIs for R/R AML during the study period were first initiated on gilteritinib (MarketScan: n=35 [67.3%], IQVIA: n=36 [70.6%]); sorafenib was the second most common FLT3-TKI (MarketScan: n=9 [17.3%], IQVIA: n=8 [15.7%]), followed by midostaurin (MarketScan: n=8 [15.4%], IQVIA: n=7 [13.7%]).
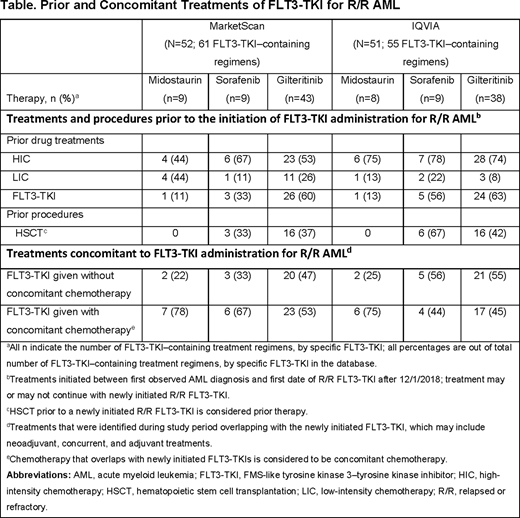
During the study period, 61 and 55 FLT3-TKI-containing regimens among 52 and 51 R/R AML patients were identified across the MarketScan and IQVIA databases, respectively. Most FLT3-TKI-containing regimens for R/R AML included gilteritinib (MarketScan: n=43 [70.5%], IQVIA: n=38 [69.1%]). Prior exposure to other FLT3-TKIs for new onset and/or R/R AML indications was observed in up to 63% of gilteritinib-, 56% of sorafenib-, and 13% of midostaurin-containing regimens (Table). Following onset of R/R AML, gilteritinib was given without concomitant chemotherapy in 47% (Marketscan: 20/43) to 55% (IQVIA: 21/38) of gilteritinib regimens; in a smaller sample of sorafenib regimens, sorafenib was given without concomitant chemotherapy in up to 56% (IQVIA: 5/9) of regimens. Of 748 patients identified in the ProMetrics database with gilteritinib claims, 714 (95%) patients were initiated on a 120-mg daily dose, with less than 5% (n=34) incurring a downward dosage titration. The median treatment duration was 150 days (95% CI: 129-172 days).
Conclusions: Among FLT3-TKI-containing regimens initiated for R/R AML after gilteritinib market approval, close to 70% included gilteritinib, an agent approved for R/R FLT3mut+ AML. Sample sizes for midostaurin- and sorafenib-containing regimens limited characterization of treatment patterns. Gilteritinib was given without concomitant chemotherapy in approximately 50% of regimens prescribed; the dose and treatment durations were similar to those previously reported by the ADMIRAL trial.
Grinblatt:Astellas: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Alexion: Speakers Bureau. Pandya:Astellas Pharma, Inc.: Current Employment. Sullivan:Astellas Pharma: Current Employment. Feng:Astellas: Current Employment. Hedlund:Astellas: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal