
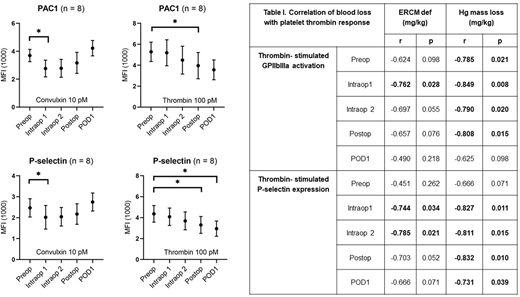
High blood loss requiring transfusion is a common complication in pediatric scoliosis surgery. Bleeding in patients undergoing spinal surgery is exacerbated by the development of coagulopathy in response to extensive activation of the acute phase response (APR). Coagulopathy is characterized by consumption of coagulation factors, inflammation, and hyperfibrinolysis. Although the administration of antifibrinolytics (e.g. tranexamic acid, TXA) has significantly reduced transfusions, patients still experience high rates of blood loss, suggesting either that TXA dosing needs optimization or that plasmin-independent aspects of coagulopathy could be driving bleeding. Major orthopedic surgeries are characterized by a reduction in platelet counts indicating the coagulopathic conditions of surgery exert a tropism on the circulating platelet population. Indeed, platelet dysfunction concomitant with trauma-induced coagulopathy has been extensively described in literature. Platelet dysfunction during adult orthopedic surgery has been observed but has not been correlated with bleeding, and platelet function in pediatric spine surgery has yet to be assessed. To determine if pediatric patients also experience platelet dysfunction, we measured platelet activation by whole blood flow cytometry during the course of scoliosis surgery in 8 pediatric patients. In parallel, circulating markers of the APR (i.e. IL-6, IL-10, IL-8), products of fibrinolysis (D-dimer), and indicators of consumptive coagulopathy (Protein C) were measured by multiplex ELISA. Samples were collected preoperatively (Preop), intraoperatively (every 2 hours, Intraop 1 and Intraop 2), immediately after closure (Postop) and the following morning (POD1). Samples were stimulated with submaximal concentrations of thrombin, convulxin (GPVI agonist, collagen receptor), and ADP. Platelet integrin activation was measured by PAC1 binding (GPIIbIIIa activation) and platelet secretion was measured by CD62p binding (P-selectin surface expression). We observed a progressive and statistically significant reduction in the platelet response (GPIIbIIIa activation and P-selectin expression) to both thrombin and convulxin stimulation during the procedure but with distinct temporal patterns. The reduction in response to thrombin became significant at Postop, remaining down even at POD1 (PAC1 max reduction, Postop 1: 32.5%, p=0.0403; P-selectin max reduction, POD1: 33.9%, p=0.016). The reduction in response to convulxin, on the other hand developed almost immediately after the initiation of surgery, starting with Intraop1 and resolving by Postop (PAC1 max reduction, Intraop 1: 25.1%, p=0.0087; P-selectin max reduction, Intraop 1: 18.3%, p=0.0369). Intra-operative responses to thrombin (both PAC1 and CD62p binding) significantly correlated with estimates of blood loss based on red blood cell mass (Estimated Red Blood Cell Mass, ERCM def) and hemoglobin mass (Hg mass loss) (see Table I below). Although we observed a consistent and statistically significant reduction in platelet number it correlated with blood loss at POD1 only, suggesting platelet function is a better predictor of blood loss than platelet count. Despite all patients receiving a bolus of TXA with continuous low dose infusion, multiplex results indicated a progressive increase in circulating D-dimer, becoming statistically significant at Postop and persisting through POD 1. Elevations in D-dimer, the byproduct of plasmin degradation of fibrin, suggest elusive fibrinolysis despite administration of TXA. Significant intraoperative elevations in IL-6, IL-10, and IL-8 and consumption of Protein C were also noted that persisted through POD1, indicating the development of coagulopathy. In conclusion, pediatric scoliosis surgery is characterized by persistent fibrinolysis platelet dysfunction, activation of the APR, and coagulopathy despite administration of the anti-fibrinolytic TXA. Future studies will focus on altering the dose of TXA to determine if an optimal dosing can be achieved that effectively inhibits fibrinolysis and answer important questions about the dependence of the APR and platelet hypofunction on the persistent fibrinolysis seen in these patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal