Background and Aims: The Asia-Pacific (APAC) Myeloma and Related Diseases Registry (MRDR) collects data on epidemiology, treatment and outcome trends for patients with multiple myeloma (MM) in participating regional countries. Prevalence of MM is expected to continue to increase with ageing populations and improvements in survival. Although some MM data are available for Asia, very few exist at regional level. Generation of 'real world evidence' on practice and outcomes is important to complement data from clinical trials. In the APAC MRDR, regional clinical experts provide local clinical context and registry oversight, and participating hospitals obtain local ethics approval and manage patient recruitment and data collection.
Methods: The APAC MRDR prospectively collects observational data on patient characteristics, diagnosis, medical history, treatment (including supportive therapies), and outcomes (overall and progression-free survival, and quality of life using the EQ-5D-5L) on newly diagnosed MM (NDMM), plasma cell leukaemia, plasmacytoma, and MGUS patients via a secure, country-specific, web-based database. Participants are reviewed 4-monthly for a minimum of 2 years. Preliminary data from October 2018 to June 2020 were analysed.
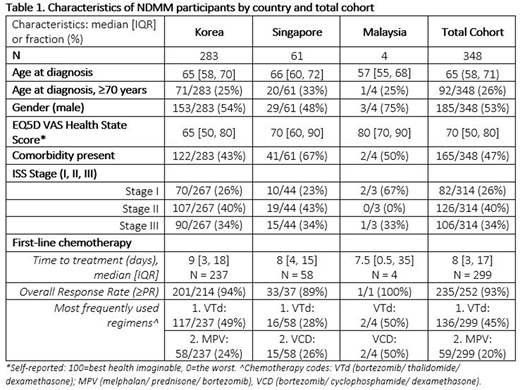
Results: 20 hospitals have ethics approval to participate and patient recruitment has commenced at 14 hospitals in Korea, Singapore, and Malaysia. Sites in Taiwan will also start recruitment in 2020, and sites in China have been identified. To date, a total of 469 patients (419 NDMM) have been enrolled, of which, data from 348 (83%) NDMM participants from Korea, Singapore, and Malaysia were available for analysis. Results are presented in Table 1.
Summary and Conclusions: The APAC MRDR is now successfully established and expanding. As data mature, these will provide important new information on the epidemiology and treatment of MM across the APAC region, and provide opportunities for regional benchmarking and collaborative research.
Spencer:Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Sanofi: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding, Speakers Bureau; Takeda: Honoraria, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Research Funding; Secura Bio: Consultancy, Honoraria; Pharmamar: Research Funding; Roche: Honoraria; Servier: Consultancy, Honoraria, Research Funding; HaemaLogiX: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal