Introduction: Emicizumab is a bi-specific, humanized, monoclonal antibody that mimics the function of activated Factor VIII (FVIII). It is approved for prophylaxis in persons of all ages with hemophilia A (HA) both with and without a FVIII inhibitor. The data on the use of emicizumab in previously untreated patients (PUPs) and minimally treated patients (MTPs) are scarce, with only one PUP reported in the pediatric clinical trials for the drug. The objective of this retrospective study was to describe our real-world institutional experience in prescribing emicizumab prophylaxis for PUPs and MTPs.
Methods: This analysis includes six patients between 1-23 months of age with severe HA prescribed emicizumab prophylaxis at Rady Children's Hospital San Diego (RCHSD) Hemophilia and Thrombosis Treatment Center between November 2017 to April 2020. Data collected through April 2020 included demographics, inhibitor history, bleeding and treatment history prior to initiation of emicizumab prophylaxis, reason for initiation of emicizumab, age, weight, and dosing regimen at the beginning of emicizumab prophylaxis, bleeding and surgical history after initiation of emicizumab, and adverse reactions to emicizumab.
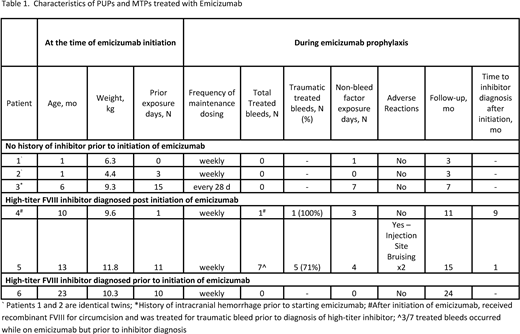
Results: Our cohort of six patients included 1 PUP and 5 MTPs with less than 20 FVIII exposure days (ED) prior to the initiation of emicizumab prophylaxis. All MTPs patients were previously treated with on-demand treatment prior to the initiation of emicizumab. Demographic and clinical characteristics are shown in Table 1. The median age and weight at initiation of emicizumab were 8 months (range: 1 to 23 months) and 9.5 kg (range: 4.4 to 11.8 kg) respectively. One patient who was treated on-demand was diagnosed with a high-titer FVIII inhibitor in the setting of a mouth bleed and started emicizumab prophylaxis after bleed management. The most common reason for initiation of emicizumab prophylaxis in non-inhibitor patients was the ability to administer medication without reliable venous access.
Following four once weekly loading doses with emicizumab, five patients continued weekly maintenance dosing and one patient continued every 28 day maintenance dosing. Patients were followed for a median of 9 months (range 3-24 months) after emicizumab initiation. Four patients, including the patient with a known FVIII inhibitor prior to starting emicizumab, had zero treated bleeds following initiation of emicizumab prophylaxis. Two patients were diagnosed with high-titer FVIII inhibitors on routine inhibitor surveillance after initiation of emicizumab prophylaxis and were the only patients who experienced treated bleeds during the study period. The majority (6 of 8) of treated bleeds were traumatic. The patient with the highest FVIII inhibitor titer experienced a disproportionately high number of the total treated bleeds though three of the seven bleeds were prior to FVIII inhibitor diagnosis. No patients required central venous catheter placement. One patient underwent circumcision with peri-operative FVIII replacement and did not have post-op bleeding. In terms of medication safety, one patient reported injection site bruising. No patient experienced severe adverse reactions including thrombotic microangiopathy, thrombosis, or clinical evidence of anti-drug neutralizing antibodies. All patients continued emicizumab prophylaxis during the study period.
Conclusions: We found that prophylaxis with emicizumab is efficacious and well-tolerated in our cohort of young children with HA under the age of 24 months both with and without high-titer FVIII inhibitors. Two patients were diagnosed with high-titer FVIII inhibitors on routine surveillance after initiation of emicizumab prophylaxis, which highlights the importance of continuing FVIII inhibitor surveillance after initiation of emicizumab. More data are needed on the use of emicizumab, particularly in PUPs and MTPs, with regards to safety, efficacy, and prevention of inhibitors in this patient population.
Thornburg:American Thrombosis and Hemostasis Network: Research Funding; National Hemophilia Foundation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer Pharmaceuticals: Research Funding; Biomarin: Consultancy, Speakers Bureau; Genentech: Speakers Bureau; NovoNordisk: Research Funding; Sanofi Genzyme: Consultancy, Other: Data Safety Monitoring Board, Research Funding; Spark Therapeutics: Consultancy; Bluebird Bio: Consultancy; Ironwood Pharmaceuticals: Consultancy, Other: Data Safety Monitoring Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal