
Background: Clinical management of ITP depends on both disease severity and risk of bleeding. Treatment typically is considered when platelet counts (PC) fall below 30,000/µL, with a target PC of ≥50,000/µL to mitigate the risk of bleeding. The standard-of-care after failure of 1st line therapy (corticosteroids or intravenous immunoglobulin) has been shifting from splenectomy and rituximab, toward an increased use of thrombopoietin receptor agonists (TPO-RAs), as supported by the recent American Society of Hematology guidelines (Neunert 2019). The TPO-RAs eltrombopag (ELT) and romiplostim (ROMI) have well understood efficacy profiles, but ELT has an FDA boxed safety warning for hepatotoxicity, necessitating hepatic function monitoring. Additionally, ELT is an orally administered chelating agent that requires dosing two hours prior to, or four hours after, meals containing polyvalent cations such as calcium or magnesium to mitigate clinically relevant effects on the pharmacokinetic profile (ELT prescribing information). In addition, ROMI, an injection, is typically is administered by a health care practitioner.
Avatrombopag (AVA) is an oral TPO-RA for patients with ITP. In clinical trials, AVA rapidly increased platelet counts as early as day 5 and maintained the median PC in the target range (50 to 150×109/L) with chronic dosing. Further, it has an exposure-adjusted safety profile generally comparable to placebo and no boxed safety warning for hepatotoxicity. AVA does not chelate polyvalent cations; therefore it is administered with food, and there are no restrictions regarding meal composition.
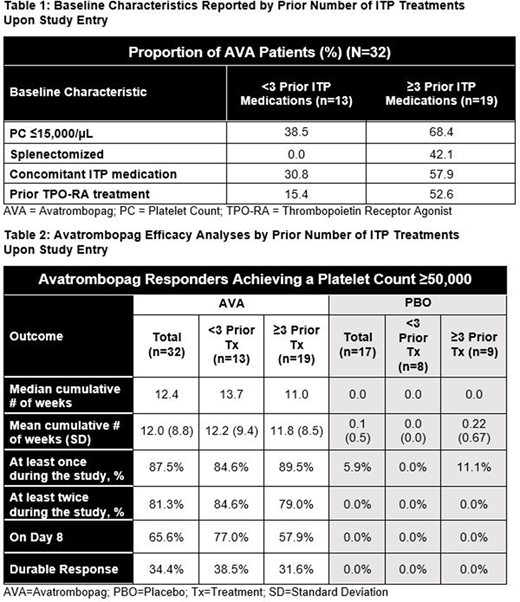
Aims: To understand how the number of prior ITP treatments impacted the efficacy of avatrombopag in a Phase 3 study.
Methods: A 6-month, multicenter, randomized, double-blind, Phase 3 study enrolled 32 AVA and 17 placebo-treated (PBO) patients with ITP. The primary endpoint was the median number of cumulative weeks of platelet count (PC) response (achieving a PC ≥50,000/µL) over the course of the study without rescue medication. Patients receiving any rescue medication during the study were deemed to be non-responders for the remainder of the study. Due to the limited number of study participants, for the post-hoc analyses reported here, the study population was segmented by whether a patient had received <3 or ≥3 prior ITP treatments upon entering the Phase 3 clinical trial. Analyses of efficacy measures were performed in these two sub-populations and are presented for hypothesis generation.
Results:Table 1 shows key differences in baseline characteristics between each group, suggesting that those receiving ≥3 prior ITP medications may have had more refractory disease. The median cumulative number of weeks of PC response for the entire population during the study was 12.4 vs. 0.0 for AVA and PBO respectively (p<0.0001), and the mean was 12.0 vs 0.1. For patients receiving <3 prior ITP treatments (n=13), the median and mean cumulative number of weeks of PC response were 13.7 and 12.2 for AVA and 0.0 and 0.0 for PBO; for patients receiving ≥3 prior ITP treatments (n=19), the median and mean were 11.0 and 11.8 weeks respectively for AVA and 0.0 and 0.2 for PBO. The proportion of AVA patients achieving a PC response at least once during the study was 87.5% for the entire population, 84.6% for those receiving <3 and 89.5% for those with ≥3 prior ITP treatments. Only 1 placebo patient ever achieved a response level PC, and that patient had received ≥3 prior ITP treatments. Similar results were noted for achieving a PC response twice or more during the study. A PC response at Day 8 was noted in 65.6% of the AVA patients and 0.0% in PBO (p<0.0001) overall, with 76.9% and 57.9% of AVA patients receiving <3 and ≥3 prior ITP treatments responding at Day 8 respectively. Finally, a durable PC response (achieving a PC ≥50,000/µL for 6 of the final 8 weeks of the study, calculated in an intent-to-treat fashion) was noted in 34.4% of AVA patients and 0.0% for PBO overall (p=0.009), while 38.5% and 31.6% of AVA patients having received <3 or ≥3 prior ITP treatments respectively.
Conclusions: In this Phase 3 study, the number of prior ITP treatments a patient had received did not seem to definitively predict PC response to AVA, though on some measures the group receiving <3 fared slightly better. These data suggest that AVA efficacy is relatively consistent, even if a patient has received multiple prior ITP therapies before AVA initiation.
Vredenburg:Dova Pharmaceuticals: Current Employment. Tian:Dova Pharmaceuticals: Current Employment. Jamieson:Dova Pharmaceuticals: Current Employment. McCrae:Rigel: Consultancy; Dova: Consultancy; Momenta Pharmaceuticals: Consultancy; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal