Background: DLBCL is a potentially curable lymphoma, yet up to 25% of older Medicare beneficiaries with DLBCL do not receive any therapy (Hamlin et al, Oncologist, 2014). Older adults with DLBCL may have vulnerabilities and require care from home health agencies. Functional impairment may compromise DLBCL therapy, but data are lacking about treatment and outcomes of home health services (HHS) recipients. There is ongoing need for accurate and efficient tools to assess function and guide therapy in geriatric patients with DLBCL, as inaccurate assessment may lead to under- and over-treatment with suboptimal outcomes. Medicare beneficiaries who receive HHS periodically undergo functional assessments using the standardized Outcome and Assessment Information Set (OASIS). OASIS scores have been recently linked to SEER-Medicare data, and can offer insights into the role of functional status in DLBCL treatment. We examined the association of functional status, measured in OASIS, and treatments and outcomes in DLBCL.
Methods: From SEER-Medicare, we selected beneficiaries diagnosed with DLBCL in 2011-2015, and identified HHS recipients who had OASIS assessments within 3 months before diagnosis or treatment. OASIS instrument measures functional status as a linear score ranging from 0 to 40; higher scores represent worse functioning. We classified the scores as mild (OASIS 0-9), moderate (10-16), and severe (17-40) impairment by tertiles of the population distribution. We examined the following outcomes: receipt of therapy (including standard [RCHOP-like] and non-standard [bendamustine, rituximab monotherapy, etc.] regimens), and among treated patients: mortality, ED visit, hospitalization, and ICU admission within 30 days from first chemotherapy. We used logistic models, reporting odds ratios (OR) with 95% confidence intervals (CI). We additionally compared overall survival (OS) between groups receiving standard and non-standard regimens in each functional category using adjusted hazard ratios (aHR) derived from Cox models. All models were adjusted for age, sex, race, DLBCL stage, and comorbidity index.
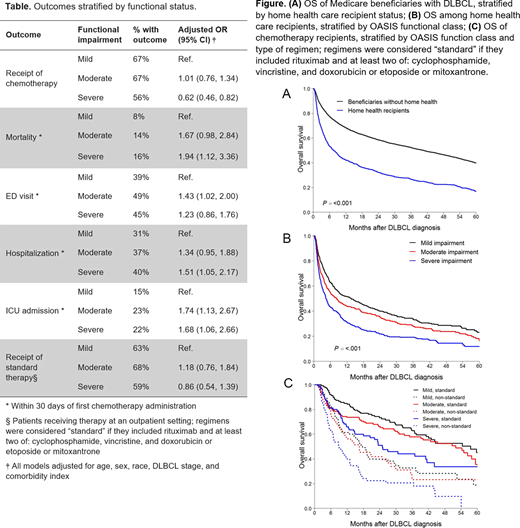
Results: Among 8,914 beneficiaries with DLBCL, 1,317 had OASIS assessments. Their characteristics included: median age 80 years, 46% men, 83% White, 53% stage 3/4 DLBCL. Median OASIS score was 13 (interquartile range, 7-18). Chemotherapy was administered to 63% of HHS recipients, compared with 75% of other beneficiaries (OR, 0.66; 95%CI, 0.57-0.76). HHS recipients were significantly more likely to experience 30-day ED visit, hospitalization, ICU admission, and mortality after chemotherapy (OR 1.24-1.52), and have shorter OS compared with other beneficiaries with DLBCL (7 vs 40 months, aHR, 1.55, 95% CI, 1.44-1.67; Fig. A).
Severe functional impairment was associated with lower odds of receiving any therapy, as well as higher rates of acute mortality, hospitalization, and ICU admission. Patients with moderate impairment had an increased risk of 30-day ED visit and ICU admission after chemotherapy (Table). OS was worse with severe functional impairment, even after adjusting for other clinical factors (aHR, 1.64, 95% CI, 1.24-2.17; Fig. B). Functional status was not significantly associated with the use of standard or non-standard regimen, and OS was better with RCHOP-like therapy in all functional groups (aHR, 0.50-0.56, P for interaction: 0.85; Fig. C).
Conclusions: In this novel population-based study using OASIS assessments to examine function as a predictor of cancer therapy, functional impairment was an independent predictor of treatment and outcomes in geriatric patients with DLBCL. We observed that a substantial proportion of patients on HHS did not receive any therapy, and worse survival in those who received attenuated regimens, independent of their functioning. Our results highlight the need for novel, less toxic strategies in this population, supporting research on emerging chemotherapy-free approaches. Clinicians should consider a dedicated functional assessment (such as a comprehensive geriatric assessment) to optimize treatment selection in DLBCL. OASIS assessments for HHS recipients are easily available to clinicians and could be incorporated into pre-chemotherapy evaluation to improve patient selection for intensive therapies, potentially avoiding under-treatment with attenuated regimens
Panagiotou:International Consulting Associates, Inc: Other: personal fees from International Consulting Associates, Inc. outside the scope of the submitted work. Olszewski:TG Therapeutics: Research Funding; Adaptive Biotechnologies: Research Funding; Spectrum Pharmaceuticals: Research Funding; Genentech, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal