Cytotoxic T cells (CTLs) and B cells engage distinct interactions in GVHD patients' blood and tissues, detectable in regular flow-cytometry screenings, by size and by double positive CD19-CD8 antibody markers (Deola, BMT 2017).
B-CTL couplets are formed by alpha-betaTCR+ CD8+ CTLs preferentially targeting CD27+ CD19+ cells displaying an activated CD80 and CD86 phenotype. Interactions may last from 5 minutes to roughly 1 hour, and release a pattern of T cell attracting chemokines, as IP10, MIG, ITAC, which are also known GVHD biomarkers.
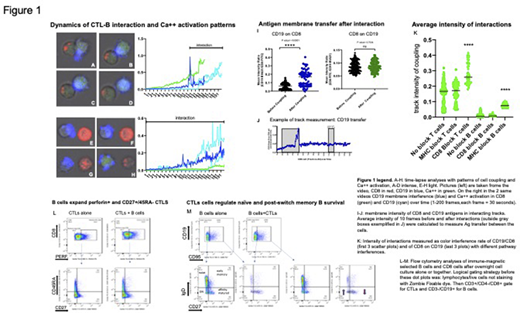
To further unravel the mechanism of this cell interaction, we built an in-vitro model where human PBMCs cells are expanded with cognate peptides and IL2 for 1-2 weeks, then immune-selected for CD8 antigen by Miltenyi microbeads negative-selection and incubated (2-18 hours) with fresh autologous CD19-B cells, immune-selected with the same method. The interactions are studied under confocal microscope video-imaging (Zeiss LSM 880+Imaris 3D analysis software) and in flow-cytometry (SymphonyA5 BD) after deep phenotype antibody staining.
The intensity of interaction, measured by fluorescence interference on cell membranes, revealed an active engagement of CD19 and CD8 antigens. CD19 antigen penetrates deeper in contacting T cells, than CD8 on B cells, and consistently with this finding, after the interactions there is an antigen exchange between cells with CD19 antigen actively transferred in CD8 cells (p value =<0.001), but not the contrary.
We already proved that this type of B-T interaction is not antigen specific in CTL-to-B direction (Deola et a, JI 2008) but to exclude cross-presentation from B to CTLs and to unravel the role of CD8, we interfered by antibody blocking of MHC class I pathway on B cells and CD8 on CTLs.
B-T cell interactions are not abolished after MHC-I or CD8 blocking, the intensity of coupling is unchanged after MHC-I block, and is higher after blocking CD8 (p value=<0.001). In particular, by blocking CD8 molecule, T cells target preferentially CD19+/CD27- cells rather than CD19+/27+ cells.
Interestingly, B cell engagement follows 2 repetitive patterns of interaction: a high intensity interaction that visually corresponds to tight coupling cells with high CD19 penetration in T cells, and a low-intensity continuous interaction, visually measurable by cells "sniffing" each other.
Both patterns correspond to diverse Calcium flux activation on T cells and B cells, suggesting functional different pathways triggered by the 2 type of interactions.
Deep phenotype flow cytometry analyses after coupling reveals distinct programs triggered by the contact in both B cells and T cells.
While after the interaction CTLs double their pool of perforin bearing effectors and their fraction of CD45RA-/CD27+ memory CTLs, CD19 preferentially undergo a deletion of IgD- CD27- (DN) cells (13,85%+/-1,1 and 22,95%+/-4,5 CD95/Fas+, respectively in B cells alone and B+CTLs, n=2) and
a rescue of affinity mature CD27+ IgD- cells (39.8%+/-25,47 and 21,2%+/- 29% CD95/Fas+ in the same groups)
CTLs are the ultimate line of "tissue attack" in GVHD and several diseases, as autoimmune diseases, cancer, viral diseases, sharing a common pathological program definable as "immune rejection". B cells are key players in immune rejection, but a link between these 2 types of cells is still unclear.
Our findings enforce the hypothesis of a program of peripheral tolerance/activation triggered directly between B cells and activated CTLs in the context of inflammation and of GVHD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal