Background: Checkpoint inhibitors, for example anti-PD-1 antibodies (e.g. nivolumab and pembrolizumab) and anti-CTLA-4 antibodies (e.g. ipilimumab), have changed the paradigm of many solid tumors although in myeloid malignancies responses have been low. Additionally, checkpoint inhibitors have a distinct toxicity profile collectively named immune-related adverse events (irAE) with unclear characterization in myeloid malignancies. Data is limited regarding predictors of irAEs and response to immunotherapy in myeloid malignancies. As we move forward in the era of immunotherapeutic agents in myeloid malignancies, better understanding of predictive biomarkers for toxicity and response is critical.
Patients and Methods: Between 2015 and 2019, patients with myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) who had been treated with checkpoint inhibitors at Moffitt Cancer Center were identified. Clinical and lab variables were obtained at date of first immunotherapy infusion. Baseline characteristics were compared using Fisher's exact test (categorical) and Mann-Whitney test (continuous). Survival estimates were calculated from date of first immunotherapy infusion. Patients receiving post induction maintenance (6) were excluded for survival analysis.
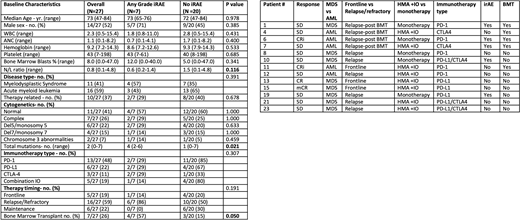
Results: A total of 28 patients, 12 MDS and 16 AML, undergoing single or combined checkpoint inhibitor therapy with either PD-1, PD-L1, or CTLA-4 targeting agents were identified. 27 patients were evaluable for irAEs. In total, 10 irAEs occurred across 7 pts with all pts having at least 1 Grade 3 event (26%). The grade 3 or higher irAEs consisted of 4 severe skin reactions (15%), 3 hepatitis (11%), and 1 immune thrombocytopenia (4%). The only other irAE noted was grade 2 hypothyroidism in patient with hepatitis.
Baseline characteristics between patients with irAEs vs without were reviewed (see table 1). Patients with irAEs appeared to have a lower neutrophil/lymphocyte (N/L) ratio (0.6 vs 1.5 p=0.116) and higher bone marrow blast % (12% vs 5% p=0.341). 0 of the patients that received immunotherapy as post induction maintenance had an irAE, compared to 33% (7/21) of non-maintenance patients. Interestingly, patients with a higher number of mutations were significantly more likely to experience irAE than those with a lower mutation burden (4 vs 1 in irAE pts vs not p=0.021). 57% of patients who received immunotherapy post bone marrow transplant (BMT) experienced an irAE, compared to 15% in the non-BMT group (p=0.050).
We then looked at potential predictors of response in the 19 evaluable patients. In total, ORR was 21% (1CR, 2Cri and 1 marrow CR). ORR +SD rate was 63%. Patients with response or stable disease (SD) following immunotherapy had a trend for lower median WBC count (1.8 vs 4.6 p=0.179) and higher platelet counts (46 vs 18 p=0.151) at baseline. Also, a higher number of total mutations was seen in response/SD patients (3 v 2 p=0.148) however this was also not significant due to sample size. In MDS patients, ORR and response/SD rate was 33% and 100% in irAE patients vs 0% and 43% in patients that lacked irAE (p=0.3 and p=0.2). There was no significant difference in ORR (33% vs 20%) or response/SD rate (60% vs 67%) in AML patients with and without irAEs. Of the patients with response to immunotherapy, 50% (2/4) harbored a TP53 mutation.
Finally, we evaluated impact of clinical and mutational factors on PFS and OS. Patients who experienced irAEs had a longer PFS compared to patients who did not; however this was not significant (5.6 vs 2.1 mo. p=0.280). OS was similar between groups (p=0.789). In MDS patients, having an irAE was associated with a statically significant improvement in PFS (9.4 vs 1.1 mo. p=0.012). Total number of mutations did not impact PFS or OS. On univariate analysis in total cohort, lower WBC and ANC were both significant independent positive predictors of PFS (p=0.032 and 0.022). On multivariate with age, disease type and BMT, low ANC only was an independent predictor of improved PFS (p=0.022).
Conclusions: In conclusion, patients with higher number of mutations and who received checkpoint inhibitors post-BMT were significantly more likely to experience an irAE. In MDS patients, patients who had an irAE had a significantly longer PFS compared to those who did not. Further studies are needed to better understand clinical predictors of irAE and response rates in patients with myeloid malignancies undergoing immunotherapy.
Padron:Incyte: Research Funding; Kura: Research Funding; Novartis: Honoraria; BMS: Research Funding. Kuykendall:Blueprint Medicines: Research Funding; BMS: Research Funding; Incyte: Research Funding; Novartis: Research Funding. Talati:AbbVie: Honoraria; Jazz: Speakers Bureau; Astellas: Speakers Bureau; BMS: Honoraria; Pfizer: Honoraria. Sweet:Stemline: Honoraria; Incyte: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria. Lancet:Abbvie: Consultancy; Agios Pharmaceuticals: Consultancy, Honoraria; Astellas Pharma: Consultancy; Celgene: Consultancy, Research Funding; Daiichi Sankyo: Consultancy; ElevateBio Management: Consultancy; Jazz Pharmaceuticals: Consultancy; Pfizer: Consultancy. Komrokji:Geron: Honoraria; Jazz: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Agios: Speakers Bureau; Abbvie: Honoraria; Incyte: Honoraria; Acceleron: Honoraria; Novartis: Honoraria. Sallman:Celgene, Jazz Pharma: Research Funding; Agios, Bristol Myers Squibb, Celyad Oncology, Incyte, Intellia Therapeutics, Kite Pharma, Novartis, Syndax: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal