The bromodomain and extraterminal domain (BET) family of proteins bind to chromatin to regulate the transcription of target genes involved in multiple pro-fibrotic pathways and is a novel therapeutic target for reducing fibrosis in myelofibrosis (MF). CPI-0610 is a unique, first-in-class, oral, small-molecule inhibitor of BET (BETi) proteins, designed to promote disease-modifying activity through selective gene regulation of key oncogenic, fibrotic, and inflammatory factors with potential to transform the standard of care in MF. Products with only one mechanism of action are approved currently for treatment of MF, Janus kinase 1/2 inhibitors (JAKi), with ruxolitinib (rux) being the standard of care for treatment-naïve MF patients. A minority of MF patients treated with rux (35%; 106 of 301) or fedratinib (37%; 35 of 96) achieved a spleen volume reduction ≥ 35% (SVR35) at 6-12 months. Disease-modifying therapeutic agents with a novel mechanism of action are needed to improve the outcomes in MF pts. Blocking BET activity with CPI-0610 inhibited aberrant maturation of megakaryocytes and decreased cytokine production in preclinical studies. In addition, synergistic antitumor activity of BETi and JAKi combination was observed in preclinical MF models. Clinical activity of CPI-0610 in combination with rux in JAKi-naïve MF patients observed in the phase 2 MANIFEST study was higher (SVR35 at wk 24: 63%) than that observed with rux alone in historical Phase 3 trials (Mascarenhas, EHA 2020).
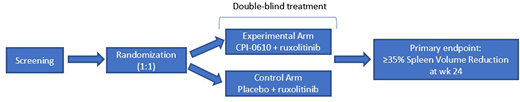
MANIFEST-2 is a global, phase 3, 1:1 randomized, double-blind, active-control study of CPI-0610 + rux vs. placebo + rux in JAKi treatment-naïve patients with primary MF, post-polycythemia-vera MF, or post-essential-thrombocythemia MF. Key eligibility criteria: DIPSS score ≥Int-1; platelet ≥100 x 109/L; spleen volume ≥ 450 cc by CT/MRI; ≥2 symptoms measurable (score ≥3) or a total symptom score (TSS) of ≥10 using the MFSAF v4.0; peripheral blast count <5%, ECOG ≤2. Approximately 310 patients (155 in each arm) will be enrolled in the study. Patient randomization will be stratified by DIPSS risk category (Intermediate-1 vs. Intermediate-2 vs. High), platelet count (> 200 × 109/L vs. 100 - 200 × 109/L), and spleen volume (≥ 1800 cm3 vs. < 1800 cm3). Double-blind treatment (CPI-0610 or matching placebo) will be administered once daily (QD) for 14 consecutive days followed by a 7-day break, which is considered 1 cycle of treatment (1 cycle = 21 days). Rux will be administered twice daily (BID) for all 21 days within each cycle. Primary endpoint: SVR35 response (≥35% reduction in spleen volume) at wk 24; key secondary endpoint: TSS50 response (≥50% reduction in TSS) at wk 24; other secondary endpoints: safety, PK, PD, bone marrow morphology/fibrosis, duration of SVR35 response, duration of TSS50 response, PFS, OS, conversion from transfusion dependence to independence, rate of RBC transfusion for the first 24 wks, hemoglobin response, peripheral proinflammatory cytokines.
Mascarenhas:Incyte, Kartos, Roche, Promedior, Merck, Merus, Arog, CTI Biopharma, Janssen, and PharmaEssentia: Other: Research funding (institution); Celgene, Prelude, Galecto, Promedior, Geron, Constellation, and Incyte: Consultancy. Harrison:Roche: Honoraria; Sierra Oncology: Honoraria; Promedior: Honoraria; AOP Orphan Pharmaceuticals: Honoraria; Gilead Sciences: Honoraria, Speakers Bureau; Incyte Corporation: Speakers Bureau; Janssen: Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau; Shire: Honoraria, Speakers Bureau; Celgene: Honoraria, Research Funding, Speakers Bureau; CTI Biopharma Corp: Honoraria, Speakers Bureau. Luptakova:Constellation Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Christo:Constellation Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Wang:Constellation Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Mertz:Constellation Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Colak:Constellation Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Shao:Constellation Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Bobba:Constellation Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Trojer:Constellation Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Senderowicz:Constellation Pharmaceuticals: Consultancy, Current equity holder in publicly-traded company, Ended employment in the past 24 months; Puma Biotechnology: Membership on an entity's Board of Directors or advisory committees. Humphrey:Constellation Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Verstovsek:Gilead: Research Funding; Promedior: Research Funding; Celgene: Consultancy, Research Funding; Roche: Research Funding; NS Pharma: Research Funding; Sierra Oncology: Consultancy, Research Funding; PharmaEssentia: Research Funding; AstraZeneca: Research Funding; ItalPharma: Research Funding; CTI Biopharma Corp: Research Funding; Incyte Corporation: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Blueprint Medicines Corp: Research Funding; Protagonist Therapeutics: Research Funding; Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal