Acute myeloid leukemia is one of the most aggressive malignant hematological disorders on worldwide basis. More than 19,000 new cases are estimated in the United States in 2020 (1.1% of all new cancer cases). The estimated death rate from AML is 11,180 that represent 56% of the new cases (1.8% of all cancer death), with an overall 5-year survival rate of 27.4% [1]. Current treatment regimens for AML include traditional chemotherapy, radiotherapy, allogeneic hematopoietic cell transplantation and targeted therapies for specific mutations in limited numbers of AML patients, all of whom still suffer from adverse effects and relapse. New broad spectrum effective and safe treatment options are urgently needed for the different types of AML.
Our strategy focused on developing a novel targeted therapy for the thyrointegrin αvβ3 receptors that are over-expressed in leukemic cells. This receptors support adhesion, engraftment, proliferation, invasion/metastasis, and angiogenesis functions of leukemic cells. The identified thyrointegrin αvβ3 antagonist fluorobenzyl Polyethylene glycol Mono-Triazole Tetraiodothyroacetic Acid (fb-PMT) binds with high affinity and specificity.
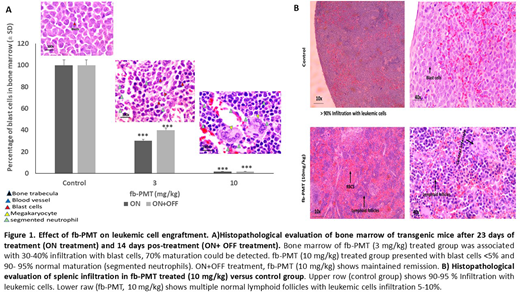
Our study showed that fb-PMT effectively suppresses the functions of AML cell line and primary cell harboring FLT3-ITD after successful engraftment in transgenic NSG-S xenograft mouse models. Daily treatment with fb-PMT at doses ranging from 1-10 mg/kg, subcutaneously for 3-4 weeks were associated with leukemogenesis regression, suppression of cancer invasion and extended survival in both models. These findings were verified using IVIS scanning and histopathological examination to evaluate the engraftment of leukemic cells in the bone marrow and other organs including, spleen, liver, lung, and brain. Furthermore, fb-PMT at 3 and 10 mg/kg, s.c. daily for 3-4 weeks exhibited significant reduction (P<0.001) of leukemic cell burden 74% and >95%, respectively. Peripheral blood smears from fb-PMT treated animals were reversed back to normal with no blast cells along with normal cell counts. Bone marrow regain its normal maturation with abundant segmented neutrophil and megakaryocytes, representing complete hematological remission along with complete suppression of leukemic cell metastasis and invasion into different organs.
To evaluate the relapse after treatment, 40 mice were maintained off treatment for further 2 weeks and IVIS scans and peripheral blood smear examination were performed on animals at end of first week and at sacrifice. The fb-PMT (10 mg/kg) off treatment has shown successful maintained remission after stopping the daily treatment as confirmed using blood smear examination, IVIS scan, and histopathological examination. Additionally, safety assessments in rodents and monkeys demonstrated safety and tolerability at multiple folds above the anticipated human doses.
Lastly, our genome-wide microarray screen demonstrated that fb-PMT works through the Hedgehog pathway and Il-21 receptor down-regulation as well as downregulation of several genes including, IGF2, TWIST1 and FYN oncogene, angiopoietin 1 (ANGPT1), angiopoietin-like 2 (ANGPTL2) and PIM1 oncogene KIT, HRAS, INH2, BCL, AKT1, IDH2, CDK4/6, TIMP1, VEGF, EGFR and PD-L1, KDM6A, EZH2.
Collectively, preclinical findings of fb-PMT warrant its clinical investigation for the effective and safe management of AML.
Reference
1. Key Statistics for Acute Myeloid Leukemia (AML). 2020; Available from: https://www.cancer.org/cancer/acute-myeloid-leukemia/about/key-statistics.html.
Mousa:Vascular Vision Pharma Co.: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal