Key Points
Adults with HR, Ph− ALL, and good MRD clearance after induction and early consolidation show promising outcomes without allo-HSCT.
Early assignment to allo-HSCT might provide long-term response to a fraction of patients showing suboptimal response to induction-1.
Abstract
The need for allogeneic hematopoietic stem cell transplantation (allo-HSCT) in adults with Philadelphia chromosome–negative (Ph−) acute lymphoblastic leukemia (ALL) with high-risk (HR) features and adequate measurable residual disease (MRD) clearance remains unclear. The aim of the ALL-HR-11 trial was to evaluate the outcomes of HR Ph− adult ALL patients following chemotherapy or allo-HSCT administered based on end-induction and consolidation MRD levels. Patients aged 15 to 60 years with HR-ALL in complete response (CR) and MRD levels (centrally assessed by 8-color flow cytometry) <0.1% after induction and <0.01% after early consolidation were assigned to receive delayed consolidation and maintenance therapy up to 2 years in CR. The remaining patients were allocated to allo-HSCT. CR was attained in 315/348 patients (91%), with MRD <0.1% after induction in 220/289 patients (76%). By intention-to-treat, 218 patients were assigned to chemotherapy and 106 to allo-HSCT. The 5-year (±95% confidence interval) cumulative incidence of relapse (CIR), overall survival (OS), and event-free survival probabilities for the whole series were 43% ± 7%, 49% ± 7%, and 40% ± 6%, respectively, with CIR and OS rates of 45% ± 8% and 59% ± 9% for patients assigned to chemotherapy and of 40% ± 12% and 38% ± 11% for those assigned to allo-HSCT, respectively. Our results show that avoiding allo-HSCT does not hamper the outcomes of HR Ph− adult ALL patients up to 60 years with adequate MRD response after induction and consolidation. Better postremission alternative therapies are especially needed for patients with poor MRD clearance. This trial was registered at www.clinicaltrials.gov as # NCT01540812.
Introduction
Therapeutic outcomes in adults with acute lymphoblastic leukemia (ALL) have substantially improved in the last decade, with complete remission (CR) and long-term overall survival (OS) rates of ∼90% and 40% to 50%, respectively.1 Better results have been attained for specific disease subtypes, such as mature B-cell acute lymphoblastic leukemia (B-ALL),2 Philadelphia chromosome (Ph)+ ALL,3 and ALL in adolescents and young adults.4,5 Improvement in these 2 subgroups was due to the frontline inclusion of anti-CD20 monoclonal antibodies6 and tyrosine kinase inhibitors,7 respectively, whereas the use of full pediatric or pediatric-inspired protocols explain the promising results in adolescents and young adults.4,5 Treatment of Ph− ALL in adults is still based on conventional multidrug chemotherapy followed or not by (usually allogeneic) hematopoietic stem cell transplantation (allo-HSCT).8,9 Prognostic factors at diagnosis, together with the level of measurable residual disease (MRD) at critical time points, are usually considered for treatment stratification.8
There is clear evidence that adult patients with standard-risk (SR) ALL at baseline and end-of-induction and/or end-of-consolidation MRD levels <0.01% are best managed with conventional chemotherapy, whereas patients with poor MRD clearance are best treated with allo-HSCT.10-13 However, is not clear whether this same principle can be applied to patients with poor risk features at diagnosis, in whom allo-HSCT has been classically considered the standard postconsolidation therapy.
The decision to transplant or not all Ph− ALL patients with high-risk (HR) features has been addressed in several prospective protocols.14 In the ALL-AR-03 protocol of the Spanish PETHEMA (Programa Español de Tratamientos en Hematología) Group postconsolidation therapy (chemotherapy or allo-HSCT) was based on both early cytological response during induction and MRD levels assessed by flow cytometry (FCM, flow-MRD) after early consolidation.15 This trial showed that sparing allo-HSCT in these patients was associated with promising results. However, this trial included patients with less stringent HR features than those usually employed to define HR ALL, and combined morphologic and MRD studies were used for evaluation of response and treatment assignment. The ALL–HR-11 trial used more stringent prognostic factors for HR definition, and the decision of postconsolidation treatment was only based on the pattern of MRD clearance, centrally assessed with a higher sensitivity and standardized technology. Herein, we report the results of this trial.
Methods
Eligibility criteria
Adolescents and adults aged 15 to 60 years with Ph− ALL and HR features were included in this prospective study. HR ALL was defined based on at least one of the following criteria: age between 30 and 60 years, white blood cell (WBC) count >30 × 109/L for B-cell precursor (BCP)-ALL or >100 × 109/L for thymic T-cell acute lymphoblastic leukemia (T-ALL), pro-B-ALL, early T-ALL or mature T-ALL, hypodiploid ALL, ALL with t(v;11q23) or KMT2A rearrangements, or with complex karyotype (≥5 unrelated clonal abnormalities). Patients were not eligible if they had an Eastern Cooperative Oncology Group performance status >2 not due to ALL, BCR-ABL1+ALL, mature B-ALL, mixed phenotype or undifferentiated AL, or lymphoblastic lymphoma. The study was activated in August 2011; patient inclusion was closed in October 2019, and follow-up was analyzed in March 2020. The protocol was approved by the institutional review board of Hospital Germans Trias i Pujol (reference PI-19-165), acting as the reference institutional review board for all participating centers, and was registered in clinicaltrials.gov (#NCT01540812). Research was conducted in accordance with the Declaration of Helsinki.
Diagnostic procedures
A diagnosis of ALL was defined as the presence of >20% lymphoblasts on morphological analysis of bone marrow (BM) specimens along with immunophenotypic study by FCM using monoclonal antibodies reactive with B-cell, T-cell, myeloid, and precursor cell-associated antigens. Early T-cell precursor (ETP) ALL was diagnosed by FCM as reported previously.16 Conventional cytogenetics was performed locally at each institution laboratory. The results of both FCM and karyotypic studies were centrally reviewed.
Minimal residual disease assessment
MRD levels in BM were centrally assessed on day 14 of induction-1 (for investigational purposes), at the end of induction-1 (weeks 5 to 6) or induction-2 in CR patients, and at the end of the third cycle of early consolidation (weeks 16 to 18) using the EuroFlow standard operating procedures and two 2-tube 8-color FCM panels for BCP-ALL and T-ALL, respectively. The Infinicyt software (Cytognos, Salamanca, Spain) was used for data analysis.17 The limit of detection and the limit of quantitation of the method were 0.2 × 10−6 and of 0.5 × 10−6, respectively. Supplemental Table 1, available on the Blood Web site, lists the antibodies used in this study for MRD evaluation.
Treatment
Before treatment, HLA typing was recommended for all patients with potential family donors, and a search for an unrelated donor was performed in CR patients lacking a histocompatible family donor. Table 1 shows the treatment schedule. Induction therapy included vincristine, prednisone, daunorubicin, and asparaginase (Escherichia coli native or pegylated, according to center availability) for 4 weeks (induction-1). The FLAG-Ida (fludarabine, cytarabine, granulocyte-colony-stimulating factor, and idarubicin) schedule was administered as intensified induction (induction-2) in patients who did not achieve CR or in those in CR with MRD levels ≥0.1% at the end of induction-1. Patients who did not achieve CR after induction-1 and induction-2 were considered failures and were excluded from the protocol but were followed by OS and event-free survival (EFS). For patients in CR and with MRD levels <0.1%, early consolidation therapy included 3 cycles with rotating cytotoxic drugs, including high-dose methotrexate, high-dose ARA-C, and high-dose asparaginase (native or pegylated, the latter capped at 3750 IU). These latter patients continued with delayed consolidation (identical to that of early consolidation) followed by standard maintenance therapy for up to 2 years in CR provided that MRD levels were <0.01% after early consolidation. Patients with CR after 2 induction cycles, those with end-induction-1 MRD ≥0.1% and patients with MRD ≥0.01% after early consolidation, were assigned to allo-HSCT if a suitable donor was available (sibling, unrelated, haploidentical, or from cord blood unit). A myeloablative regimen, including fractionated total body irradiation, was recommended as the conditioning regimen in patients with a good clinical status and under 55 years of age, whereas a nonmyeloablative regimen with fludarabine and melphalan was suggested in patients in poor clinical condition or older than 55 years. Central nervous system (CNS) prophylaxis consisted of triple intrathecal chemotherapy (TIT) and was given during induction, consolidation, and maintenance for a total of 14 administrations. TIT was also recommended prior to and after HSCT. Patients with CNS involvement at diagnosis received TIT every 3 to 4 days until the cerebrospinal fluid was clear in the last 2 samples of a minimum number of 5 lumbar punctures. The patients then followed the regular CNS prophylaxis. No CNS radiotherapy was given. Rituximab was not given to patients in this trial. Dose reductions for patients older than 50 years included methotrexate 1.5 g/m2, cytarabine 1000 mg/m2/12 hours, and pegylated asparaginase 1000 IU/m2 (Table 1). Asparaginase activity was not assessed in this study. Toxicity was registered in the induction and early consolidation phases of treatment.
PETHEMA ALL-HR-11 trial: chemotherapy schedule
| Phase . | Route . | Dose . | Days . |
|---|---|---|---|
| Induction-1* | |||
| Vincristine (maximum 2 mg) | IV | 1.5 mg/m2 | 1, 8, 15, 22 |
| Daunorubicin | IV | 45 mg/m2 | 1, 8, 15, 22 |
| Prednisone | IV | 60 mg/m2 | 1 to 14 |
| IV | 30 mg/m2 | 15 to 21 | |
| IV | 15 mg/m2 | 22 to 28 | |
| Native ASP† or | IV | 10 000 IU/m2 | 16-20, 23-27 |
| PEG-ASP†,‡ | IV | 2000 IU/m2 | 15 |
| TIT§ | IT | 1, 22 | |
| Induction-2‖ | |||
| Idarubicin | IV | 12 mg/m2 | 1, 3, 5 |
| Fludarabine | IV | 30 mg/m2 | 1-5 |
| ARA-C | IV | 2000 mg/m2 | 1-5 |
| TIT§ | IT | 7 | |
| Early consolidation-1 | |||
| Vincristine | IV | 2 mg | 1, 8 |
| Dexamethasone | IV/PO | 20 mg/m2 | 1-5 |
| IV/PO | 10 mg/m2 | 6 | |
| IV/PO | 5 mg/m2 | 7 | |
| IV/PO | 2.5 mg/m2 | 8 | |
| Methotrexate¶ | IV | 3 or 5 g/m2 | 1 |
| Native ASP† or | IV | 20 000 IU/m2 | 3 |
| PEG-ASP†,‡ | IV | 2000 IU/m2 | 3 |
| TIT§ | IT | 1 | |
| Early consolidation-2 | |||
| Dexamethasone | IV/PO | 20 mg/m2 | 1-5 |
| IV/PO | 10 mg/m2 | 6 | |
| IV/PO | 5 mg/m2 | 7 | |
| IV/PO | 2.5 mg/m2 | 8 | |
| ARA-C | IV | 2000 mg/m2/12h | 1, 2 |
| Native ASP† or | IV | 20 000 IU/m2 | 3 |
| PEG-ASP†,‡ | IV | 2000 IU/m2 | 3 |
| TIT§ | IT | 4 | |
| Early consolidation-3 | |||
| Vincristine | IV | 2 mg | 1, 8 |
| Dexamethasone | IV/PO | 20 mg/m2 | 1-5 |
| IV/PO | 10 mg/m2 | 6 | |
| IV/PO | 5 mg/m2 | 7 | |
| IV/PO | 2.5 mg/m2 | 8 | |
| Methotrexate¶ | IV | 3 or 5 g/m2 | 1 |
| Native ASP† or | IV | 20 000 IU/m2 | 3 |
| PEG-ASP†,‡ | IV | 2000 IU/m2 | 3 |
| TIT§ | IT | 1 | |
| Delayed consolidation-1# | |||
| Vincristine | IV | 2 mg | 1, 8 |
| Dexamethasone | IV/PO | 20 mg/m2 | 1-5 |
| IV/PO | 10 mg/m2 | 6 | |
| IV/PO | 5 mg/m2 | 7 | |
| IV/PO | 2.5 mg/m2 | 8 | |
| Methotrexate¶ | IV | 3 or 5 g/m2 | 1 |
| Native ASP† or | IV | 20 000 IU/m2 | 3 |
| PEG-ASP†,‡ | IV | 2000 IU/m2 | 3 |
| TIT§ | IT | 1 | |
| Delayed consolidation-2# | |||
| Dexamethasone | IV/PO | 20 mg/m2 | 1-5 |
| IV/PO | 10 mg/m2 | 6 | |
| IV/PO | 5 mg/m2 | 7 | |
| IV/PO | 2.5 mg/m2 | 8 | |
| ARA-C | IV | 2000 mg/m2/12 h | 1, 2 |
| Native ASP† or | IV | 20 000 IU/m2 | 3 |
| PEG-ASP†,‡ | IV | 2000 IU/m2 | 3 |
| TIT§ | IT | 4 | |
| Delayed consolidation-3# | |||
| Vincristine | IV | 2 mg | 1, 8 |
| Dexamethasone | IV/PO | 20 mg/m2 | 1-5 |
| IV/PO | 10 mg/m2 | 6 | |
| IV/PO | 5 mg/m2 | 7 | |
| IV/PO | 2.5 mg/m2 | 8 | |
| Methotrexate¶ | IV | 3 or 5 g/m2 | 1 |
| Native ASP† or | IV | 20 000 IU/m2 | 3 |
| PEG-ASP†,‡ | IV | 2000 IU/m2 | 3 |
| TIT§ | IT | 1 | |
| Maintenance-1** | |||
| Mercaptopurine | PO | 50 mg/m2 | Daily |
| Methotrexate | IM | 20 mg/m2 | Weekly |
| Reinductions†† | |||
| Vincristine | IV | 2 mg | 1 |
| Prednisone | PO | 60 mg | 1-7 |
| TIT§ | IT | 1 | |
| Maintenance-2‡‡ | |||
| Mercaptopurine | PO | 50 mg/m2 | Daily |
| Methotrexate | IM | 20 mg/m2 | Weekly |
| Phase . | Route . | Dose . | Days . |
|---|---|---|---|
| Induction-1* | |||
| Vincristine (maximum 2 mg) | IV | 1.5 mg/m2 | 1, 8, 15, 22 |
| Daunorubicin | IV | 45 mg/m2 | 1, 8, 15, 22 |
| Prednisone | IV | 60 mg/m2 | 1 to 14 |
| IV | 30 mg/m2 | 15 to 21 | |
| IV | 15 mg/m2 | 22 to 28 | |
| Native ASP† or | IV | 10 000 IU/m2 | 16-20, 23-27 |
| PEG-ASP†,‡ | IV | 2000 IU/m2 | 15 |
| TIT§ | IT | 1, 22 | |
| Induction-2‖ | |||
| Idarubicin | IV | 12 mg/m2 | 1, 3, 5 |
| Fludarabine | IV | 30 mg/m2 | 1-5 |
| ARA-C | IV | 2000 mg/m2 | 1-5 |
| TIT§ | IT | 7 | |
| Early consolidation-1 | |||
| Vincristine | IV | 2 mg | 1, 8 |
| Dexamethasone | IV/PO | 20 mg/m2 | 1-5 |
| IV/PO | 10 mg/m2 | 6 | |
| IV/PO | 5 mg/m2 | 7 | |
| IV/PO | 2.5 mg/m2 | 8 | |
| Methotrexate¶ | IV | 3 or 5 g/m2 | 1 |
| Native ASP† or | IV | 20 000 IU/m2 | 3 |
| PEG-ASP†,‡ | IV | 2000 IU/m2 | 3 |
| TIT§ | IT | 1 | |
| Early consolidation-2 | |||
| Dexamethasone | IV/PO | 20 mg/m2 | 1-5 |
| IV/PO | 10 mg/m2 | 6 | |
| IV/PO | 5 mg/m2 | 7 | |
| IV/PO | 2.5 mg/m2 | 8 | |
| ARA-C | IV | 2000 mg/m2/12h | 1, 2 |
| Native ASP† or | IV | 20 000 IU/m2 | 3 |
| PEG-ASP†,‡ | IV | 2000 IU/m2 | 3 |
| TIT§ | IT | 4 | |
| Early consolidation-3 | |||
| Vincristine | IV | 2 mg | 1, 8 |
| Dexamethasone | IV/PO | 20 mg/m2 | 1-5 |
| IV/PO | 10 mg/m2 | 6 | |
| IV/PO | 5 mg/m2 | 7 | |
| IV/PO | 2.5 mg/m2 | 8 | |
| Methotrexate¶ | IV | 3 or 5 g/m2 | 1 |
| Native ASP† or | IV | 20 000 IU/m2 | 3 |
| PEG-ASP†,‡ | IV | 2000 IU/m2 | 3 |
| TIT§ | IT | 1 | |
| Delayed consolidation-1# | |||
| Vincristine | IV | 2 mg | 1, 8 |
| Dexamethasone | IV/PO | 20 mg/m2 | 1-5 |
| IV/PO | 10 mg/m2 | 6 | |
| IV/PO | 5 mg/m2 | 7 | |
| IV/PO | 2.5 mg/m2 | 8 | |
| Methotrexate¶ | IV | 3 or 5 g/m2 | 1 |
| Native ASP† or | IV | 20 000 IU/m2 | 3 |
| PEG-ASP†,‡ | IV | 2000 IU/m2 | 3 |
| TIT§ | IT | 1 | |
| Delayed consolidation-2# | |||
| Dexamethasone | IV/PO | 20 mg/m2 | 1-5 |
| IV/PO | 10 mg/m2 | 6 | |
| IV/PO | 5 mg/m2 | 7 | |
| IV/PO | 2.5 mg/m2 | 8 | |
| ARA-C | IV | 2000 mg/m2/12 h | 1, 2 |
| Native ASP† or | IV | 20 000 IU/m2 | 3 |
| PEG-ASP†,‡ | IV | 2000 IU/m2 | 3 |
| TIT§ | IT | 4 | |
| Delayed consolidation-3# | |||
| Vincristine | IV | 2 mg | 1, 8 |
| Dexamethasone | IV/PO | 20 mg/m2 | 1-5 |
| IV/PO | 10 mg/m2 | 6 | |
| IV/PO | 5 mg/m2 | 7 | |
| IV/PO | 2.5 mg/m2 | 8 | |
| Methotrexate¶ | IV | 3 or 5 g/m2 | 1 |
| Native ASP† or | IV | 20 000 IU/m2 | 3 |
| PEG-ASP†,‡ | IV | 2000 IU/m2 | 3 |
| TIT§ | IT | 1 | |
| Maintenance-1** | |||
| Mercaptopurine | PO | 50 mg/m2 | Daily |
| Methotrexate | IM | 20 mg/m2 | Weekly |
| Reinductions†† | |||
| Vincristine | IV | 2 mg | 1 |
| Prednisone | PO | 60 mg | 1-7 |
| TIT§ | IT | 1 | |
| Maintenance-2‡‡ | |||
| Mercaptopurine | PO | 50 mg/m2 | Daily |
| Methotrexate | IM | 20 mg/m2 | Weekly |
ASP, asparaginase, IM, intramuscular administration; IT, intrathecal administration; PO, oral administration; TIT, triple intrathecal therapy.
Prephase with prednisone 60 mg/m2 and triple intrathecal therapy were given for a maximum of 1 wk, while ALL was fully characterized.
50% dose reduction in patients over 50 y old.
3750 IU (1 vial) in patients with body surface area over 1.8 m2.
Triple intrathecal therapy with methotrexate (15 mg), ARA-C (30 mg), and hydrocortisone (20 mg).
Only for patients who did not achieve CR after induction-1 or whose MRD level was ≥0.1%.
3 g/m2 for BCP-ALL, 5 g/m2 for T-ALL. Dose reduction to 1.5 g/m2 for patients aged >50 y.
Only for patients assigned to receive chemotherapy.
Until completing 1 y from the date of the complete remission.
Every month.
During the second year from the date of the complete remission.
Criteria for response, relapse, and follow-up
CR was defined as the absence of extramedullary disease, neutrophils >1 × 109/L, platelets >100 × 109/L, and <5% BM blast cells. Poor immunological response was defined as an MRD level ≥0.1% at the end of induction-1 and ≥0.01% at the end of early consolidation. Resistant disease (RD) was defined as persistence of leukemia in patients surviving induction. Early death (ED) was defined as death occurring before fulfilling the criteria for CR or RD. Relapse was defined as disease recurrence at any site after achieving CR. OS was measured from the time of entry in the protocol to the time of death or last follow-up. EFS was registered in all patients from diagnosis to last follow-up, considering ED, RD, relapse, or death by any cause as events. The cumulative incidence of relapse (CIR) was calculated from the date of first CR to the date of relapse, considering nonrelapse mortality (NRM) as a competing event. Toxicity was evaluated according to the Common Terminology Criteria for Adverse Events (v 4.0) criteria.
Statistical analyses
The primary study objective was OS according to the 2 postinduction treatment options evaluated by intention to treat (ITT). Secondary objectives were the rates of morphologic and MRD response, EFS, and CIR either by ITT or by the treatment actually given, as well as comparison with the outcomes of similar patients included in the ALL-AR-03 trial. The main clinical and hematological variables in the whole series, as well as in the different treatment subgroups, were compared by median test (continuous variables) and the Pearson or Fisher’s exact tests (categorical variables). OS and EFS curves were plotted by the Kaplan-Meier method and compared by the log-rank test. CIR was estimated using cumulative incidence functions by competing risks analysis. Gray’s test was used for comparison of CIR curves. Multivariable analyses for OS and CIR were performed using the Cox proportional hazards regression model and the Fine and Gray model, respectively. No imputation method was used for missing data. Data collection and statistical analyses were performed at the PETHEMA Data Center for ALL using SPSS (v.24) and R (v.3.5.2) software. Two-sided values of P < .05 were considered statistically significant.
Results
Patient entry
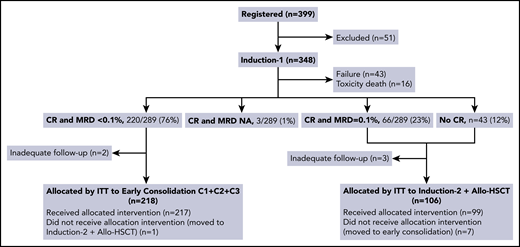
From August 2011 to October 2019, 399 adolescent and adult patients with HR, Ph− ALL from 51 Spanish hospitals were registered, 348 of whom entered the study and were included in the analysis. The reasons for noneligibility among the remaining 51 patients were lymphoblastic lymphoma (n = 26), age >60 years (n = 6), mixed phenotype AL (n = 6), Ph+ ALL (n = 4), mature B-ALL (n = 4), human T-cell lymphotropic virus type-1–related adult T-cell leukemia-lymphoma (n = 1), dendritic cell leukemia (n = 1), absence of HR criteria (n = 1), severe psychiatric disorder (n = 1), and major protocol deviation (n = 1) (Figure 1; supplemental Figure 1).
Flowchart of assignations of postinduction treatment by ITT. NA, not available; C1, first consolidation cycle; C2, second consolidation cycle; C3, third consolidation cycle.
Flowchart of assignations of postinduction treatment by ITT. NA, not available; C1, first consolidation cycle; C2, second consolidation cycle; C3, third consolidation cycle.
Pretreatment characteristics, response to induction therapy, and postinduction assignment of patients
The main demographic and clinical characteristics are summarized in Table 2. Of the 348 patients, 212 (61%) were men. The median age at the start of treatment was 40 years (range, 15 to 60 years); 262 (75%) patients were 30 to 60 years of age. The blast cell lineage was B cell in 241 (69%) patients and T cell in 107 (31%, 23 diagnosed with ETP ALL).16 Information on CD20 expression in blast cells was available in 184/241 patients with BCP-ALL, of whom 70 (38%) were CD20+ (defined as CD20 expression in >20% of blasts). Extramedullary disease was observed in 90/345 patients (26%), with the CNS (n = 38) and mediastinum (n = 51) being the most frequent sites involved. Cytogenetic findings included t(v;11q23) rearrangements in 24 (7%) patients, hypodiploidy in 10 (3%), t(1;19) in 12 (3%), and complex karyotype in 10 (3%), among others.
Patient characteristics of all eligible patients, and of patients assigned to chemotherapy or to allo-HSCT by ITT
| Characteristic . | Whole series (n = 348) . | Chemotherapy (n = 218) . | Allo-HSCT (n = 106) . |
|---|---|---|---|
| Age, y | |||
| Median, range | 40 (15-60) | 39 (15-60) | 39 (16-60) |
| ≥30 y (%) | 262 (75) | 163 (75) | 76 (72) |
| Sex (%) | |||
| Male | 212 (61) | 128 (59) | 71 (67) |
| Female | 136 (39) | 90 (41) | 35 (33) |
| Extramedullary involvement | |||
| Mediastinum (%) | 51/334 (15) | 35/208 (17) | 15/103 (15) |
| CNS (%) | 38/331 (11) | 19/211 (9)† | 16/99 (16)† |
| Testicle (%) | 2/194 (1) | 1/119 (1) | 1/63 (2) |
| Other (%) | 21/328 (6)* | 12/207 (6) | 6/98 (6) |
| WBC count, ×109/L | |||
| Median, range | 13.5 (0.2-638) | 14.7 (0.2-564) | 13.2 (0.6-638) |
| WBC >30 × 109/L (%) | 138 (40) | 85 (39) | 43 (41) |
| WBC >100 × 109/L (%) | 64 (18) | 37 (17) | 25 (24) |
| Immunophenotype (%) | |||
| BCP | 241 (69) | 147 (67) | 74 (70) |
| Early pre-B | 39 (11) | 15 (7) | 19 (18) |
| Common | 158 (46) | 99 (45) | 48 (45) |
| Pre-B | 43 (12) | 33 (15) | 7 (7) |
| B, nonspecified | 1 (0.5) | 0 | 0 |
| T-ALL | 107 (31) | 71 (33) | 32 (30) |
| Early pre-T | 23 (7) | 5 (2)‡ | 17 (16)‡ |
| Pre-T | 19 (5.5) | 15 (7) | 3 (3) |
| Cortical | 42 (12) | 34 (16) | 6 (5) |
| Mature | 18 (5) | 13 (6) | 5 (5) |
| T, nonspecified | 5 (1) | 4 (2) | 1 (1) |
| Cytogenetics (%) | |||
| Normal | 95/343 (28) | 60/214 (28) | 27/105 (26) |
| Abnormal | 157/343 (46) | 96/214 (45) | 50/105 (48) |
| Hyperdiploidy | 7 (2) | 7 (7) | 0 |
| Hypodiploidy | 10 (3) | 7 (7) | 2 (4) |
| t(v;11q23)/KMT2A | 24 (7) | 9 (10)§ | 13 (26)§ |
| t(1;19) | 12 (3) | 11 (12)§ | 1 (2)§ |
| t(12;21)/ETV6-RUNX1 | 2 (1) | 0 | 1 (2) |
| Other translocations | 18 (5) | 12 (13) | 4 (8) |
| del (9p) | 7 (2) | 7 (7) | 0 |
| del (12p) | 7 (2) | 2 (2) | 3 (6) |
| del (6q) | 4 (1) | 3 (3) | 1 (2) |
| Other deletions | 13 (4) | 8 (8) | 4 (8) |
| Complex (≥5 abnormalities) | 10 (3) | 6 (6) | 3 (6) |
| Other abnormalities | 43 (13) | 24 (25) | 18 (36) |
| Not evaluable | 91/343 (26) | 58/214 (27) | 28/105 (26) |
| Normal, <20 metaphases | 31 (9) | 21 (10) | 9 (8) |
| No growth | 60 (17) | 37 (17) | 19 (18) |
| Characteristic . | Whole series (n = 348) . | Chemotherapy (n = 218) . | Allo-HSCT (n = 106) . |
|---|---|---|---|
| Age, y | |||
| Median, range | 40 (15-60) | 39 (15-60) | 39 (16-60) |
| ≥30 y (%) | 262 (75) | 163 (75) | 76 (72) |
| Sex (%) | |||
| Male | 212 (61) | 128 (59) | 71 (67) |
| Female | 136 (39) | 90 (41) | 35 (33) |
| Extramedullary involvement | |||
| Mediastinum (%) | 51/334 (15) | 35/208 (17) | 15/103 (15) |
| CNS (%) | 38/331 (11) | 19/211 (9)† | 16/99 (16)† |
| Testicle (%) | 2/194 (1) | 1/119 (1) | 1/63 (2) |
| Other (%) | 21/328 (6)* | 12/207 (6) | 6/98 (6) |
| WBC count, ×109/L | |||
| Median, range | 13.5 (0.2-638) | 14.7 (0.2-564) | 13.2 (0.6-638) |
| WBC >30 × 109/L (%) | 138 (40) | 85 (39) | 43 (41) |
| WBC >100 × 109/L (%) | 64 (18) | 37 (17) | 25 (24) |
| Immunophenotype (%) | |||
| BCP | 241 (69) | 147 (67) | 74 (70) |
| Early pre-B | 39 (11) | 15 (7) | 19 (18) |
| Common | 158 (46) | 99 (45) | 48 (45) |
| Pre-B | 43 (12) | 33 (15) | 7 (7) |
| B, nonspecified | 1 (0.5) | 0 | 0 |
| T-ALL | 107 (31) | 71 (33) | 32 (30) |
| Early pre-T | 23 (7) | 5 (2)‡ | 17 (16)‡ |
| Pre-T | 19 (5.5) | 15 (7) | 3 (3) |
| Cortical | 42 (12) | 34 (16) | 6 (5) |
| Mature | 18 (5) | 13 (6) | 5 (5) |
| T, nonspecified | 5 (1) | 4 (2) | 1 (1) |
| Cytogenetics (%) | |||
| Normal | 95/343 (28) | 60/214 (28) | 27/105 (26) |
| Abnormal | 157/343 (46) | 96/214 (45) | 50/105 (48) |
| Hyperdiploidy | 7 (2) | 7 (7) | 0 |
| Hypodiploidy | 10 (3) | 7 (7) | 2 (4) |
| t(v;11q23)/KMT2A | 24 (7) | 9 (10)§ | 13 (26)§ |
| t(1;19) | 12 (3) | 11 (12)§ | 1 (2)§ |
| t(12;21)/ETV6-RUNX1 | 2 (1) | 0 | 1 (2) |
| Other translocations | 18 (5) | 12 (13) | 4 (8) |
| del (9p) | 7 (2) | 7 (7) | 0 |
| del (12p) | 7 (2) | 2 (2) | 3 (6) |
| del (6q) | 4 (1) | 3 (3) | 1 (2) |
| Other deletions | 13 (4) | 8 (8) | 4 (8) |
| Complex (≥5 abnormalities) | 10 (3) | 6 (6) | 3 (6) |
| Other abnormalities | 43 (13) | 24 (25) | 18 (36) |
| Not evaluable | 91/343 (26) | 58/214 (27) | 28/105 (26) |
| Normal, <20 metaphases | 31 (9) | 21 (10) | 9 (8) |
| No growth | 60 (17) | 37 (17) | 19 (18) |
Including pericardium and pleura (n = 4), pericardium (n = 1), pleura (n = 2), bone (n = 4), bone and pleura (n = 1), skin (n = 4), kidney (n = 2), kidney and pancreas (n = 1), ovary (n = 1), and muscle (n = 1).
P = .063.
Early pre–T- vs nonearly T-cell precursor: 5 vs 17; 66 vs 15 (P < .001).
t(v;11q23) vs t(1;19) vs other: 9 vs 13; 11 vs 1; 76 vs 36 (P = .007).
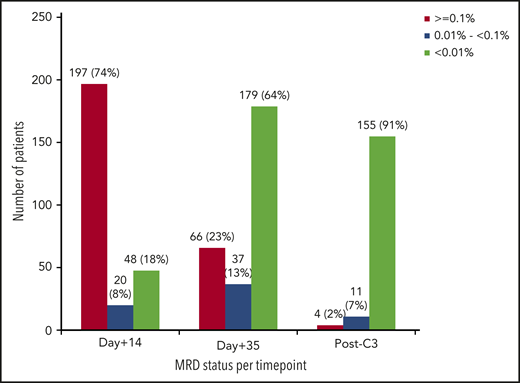
The main outcome results after induction-1 included ED in 16 patients (5%), failure in 43 (12%), and CR in 289 (83%, 220 with MRD <0.1%, 66 with MRD ≥0.1%, and 3 with MRD not assessed; Figure 1). Deeper MRD response (<0.01% after induction-1) was achieved by 179/282 (63%) of patients achieving CR with available MRD assessment (Figure 2). Among the patients with t(v;11q23)-rearranged ALL (n = 24), 18 (75%) achieved CR after induction-1 and 7/18 (39%) had MRD <0.01%. In turn, 11/23 (48%) with ETP-ALL achieved CR after induction-1 and 4/11 (36%) had MRD <0.01%. Forty-eight out of 265 patients (18%) in whom the MRD study could be performed on day 14 of induction therapy as an exploratory assessment showed a MRD level <0.01% (Figure 2). Induction-2 was administered to 99 patients (41 after failure of induction-1 and 58 in CR but MRD ≥0.1%). Of 58 patients in CR but with MRD ≥0.1%, 3 did not complete induction-2 at the time of analysis, 7 died during therapy, 2 were refractory (lost previous CR), 2 experienced relapse before the first consolidation cycle, and the remaining 44 maintained CR (MRD was assessed in 42 patients, <0.01% in 29). Of 41 patients refractory to induction-1, 1 was on induction-2 at the time of analysis, 5 died during therapy, 7 were refractory, and the remaining 28 achieved CR. Two patients receiving blinatumomab after induction-2 were censored at the time of initiation of blinatumomab. To summarize, 315/348 patients (91%) attained CR after induction-1+2. Figure 1 shows the flowchart of the assignment of postinduction treatment by ITT, and supplemental Figure 1 shows the treatment received by the patients.
MRD status after induction-1 and early consolidation therapy. D+14, mid–induction-1; d+35, end–induction-1 (around week 5); post-C3, after the third cycle of early consolidation.
MRD status after induction-1 and early consolidation therapy. D+14, mid–induction-1; d+35, end–induction-1 (around week 5); post-C3, after the third cycle of early consolidation.
Of the 220 patients assigned to chemotherapy by ITT (because of morphologic CR after induction-1 or to CR and MRD <0.1%), 218 were finally evaluable (Figure 1). The 2 remaining patients were excluded because of inadequate follow-up (1 withdrew from the study post–induction-1 and the remaining patient was lost to follow-up immediately after induction-1). Two hundred seventeen patients received the allocated early consolidation (the remaining patient erroneously received induction-2 therapy and allo-HSCT). Delayed consolidation was given to 145 patients. Relapse (n = 22), death by toxicity (n = 2), withdrawal due to toxicity (n = 2), ongoing treatment (n = 12), protocol deviation (n = 29), and allo-HSCT based on post-early consolidation MRD levels ≥0.01% (n = 5) were the main reasons for not receiving delayed consolidation. The protocol deviations (n = 29) were as follows: immunotherapy with blinatumomab (n = 7), allogeneic HSCT despite an MRD level <0.01% (n = 9), delayed consolidation despite MRD ≥ 0.01% (n = 8), other chemotherapy diff-erent from that of the protocol (n = 1), autologous HSCT (n = 1), and noncompleted delayed consolidation (n = 3). FCM MRD levels <0.01% at the end of early consolidation were observed in 155/170 evaluable patients (91%) (Figure 2). Maintenance therapy was given to 125 patients, 64 of whom completed treatment. The remaining 61 patients relapsed (n = 26) were withdrawn from the treatment because of toxicity (n = 2) or were receiving therapy at the time of analysis (n = 33). Fifty-five patients were alive in first CR, whereas the remaining 9 patients relapsed off-therapy (supplemental Figure 1).
One hundred six out of 109 patients assigned to allo-HSCT (because of absence of CR after induction-1 or to CR but with MRD ≥0.1%) were finally evaluable. The 3 remaining patients were excluded because of lack of adequate follow-up (2 withdrew from the trial, and 1 was lost to follow-up just after induction-1). Of these, 99 received the allocated intervention, treatment within the chemotherapy arm was the reason for not receiving the allocated intervention in the remaining 7 patients (Figure 1). Seventy out of 99 patients who received induction-2 were treated with the first consolidation cycle. ED (n = 12), resistance (n = 9), relapse (n = 2), protocol deviation (treatment with blinatumomab, n = 2), or ongoing treatment (n = 4) were the main reasons for not receiving this cycle. Allo-HSCT was performed in 57/70 patients. The reasons for not performing allo-HST were relapse (n = 5), death by toxicity (n = 1), transfer of patients to the chemotherapy arm (n = 5), and waiting for transplantation (n = 2). It is of note that 5 additional patients were transplanted because of post early consolidation MRD levels ≥0.01%. Thirty-two patients from this group are alive in first CR.
CIR, OS, and event-free survival
Whole series
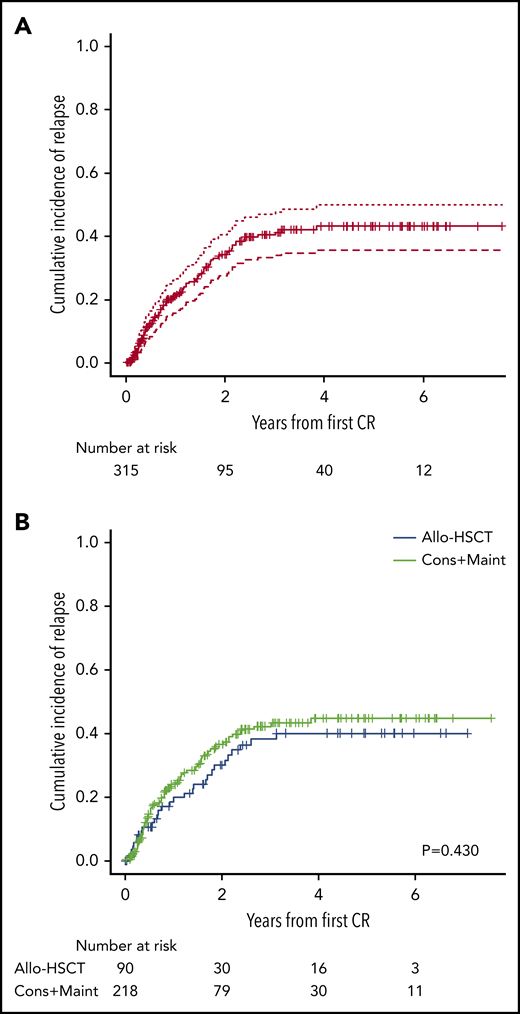
Of the 315 patients who attained CR, 89 (28%) relapsed (BM [n = 66], CNS [n = 9], BM and CNS [n = 5], BM, CNS and testicular [n = 1], BM and testicular [n = 2], BM and skin [n = 1]), BM and mediastinum [n = 1], lymph nodes [n = 2], and extramedullary, nonspecified [n = 2]). Relapses occurred during treatment in 63 patients and off-therapy in 26. The 5-year CIR was 43% (95% confidence interval [CI], 36% to 50%) (Figure 3A), with no differences according to phenotype (BCP, 41% [32% to 50%], T-ALL, 48% [35% to 60%]). Patients with ETP-ALL showed a higher (albeit nonsignificant) CIR than the remaining T-ALL cases (64% [31% to 84%] vs 39% [26% to 53%]).
Cumulative incidence of relapse. (A) Cumulative incidence plot for the whole series. (B) Cumulative incidence plots for patients receiving consolidation and maintenance (Cons+Maint) and for patients submitted to allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Cumulative incidence of relapse. (A) Cumulative incidence plot for the whole series. (B) Cumulative incidence plots for patients receiving consolidation and maintenance (Cons+Maint) and for patients submitted to allogeneic hematopoietic stem cell transplantation (allo-HSCT).
After a median follow-up of the cohort of 2.4 years (range: 0.2-7.7 years), 132 patients died and 216 remain alive, with a median OS of 4.2 years and a projected 5-year survival probability of 49% (42% to 56%) (Figure 4A). The main cause of death was relapsed disease (n = 78). Deaths attributable to treatment toxicity (n = 48) occurred in induction (n = 28), during early consolidation (n = 5), after allo-HSCT (n = 11), and during delayed consolidation (n = 4). The cause of death in the remaining 6 cases was a second neoplasia. A trend for poorer OS for ETP-ALL vs other T-ALL subtypes (31% [8% to 59%] vs 66% [52% to 76%], respectively, P = .06) was observed (supplemental Figure 2A). Patients with 11q23 rearrangements also showed poorer survival than the remaining BCP-ALL patients (37% [18% to 55%] vs 49% [40% to 58%], respectively, P = .04) (supplemental Figure 2B). The median EFS was 2 years (95% CI: 1.5 to 2.5 years) and the 5-year EFS probability was 40% (34% to 47%) (Figure 4B), being significantly poorer for ETP-ALL vs other T-ALL (20% [6% to 41%] vs 52% [38% to 64%], P = .04). On the contrary, no significant differences for EFS were observed on comparison of t(v;11q23)-rearranged ALL vs other BCP-ALL patients. A high WBC count, adverse cytogenetics (t[v;11q23], hypodiploidy and complex karyotype) and MRD ≥0.01% after induction-1 were found to be the main prognostic factors for OS in the multivariable analyses, whereas a high WBC count was associated with an increased CIR (Table 3). It is of note that younger patients (15 to 29 years), with a high CIR by univariable analysis, showed poorer prognostic features at baseline than patients ≥30 years of age (significantly higher WBC count and T-cell phenotype). On the other hand, there were no differences in OS and CIR according to the expression of CD20 in blast cells (data not shown).
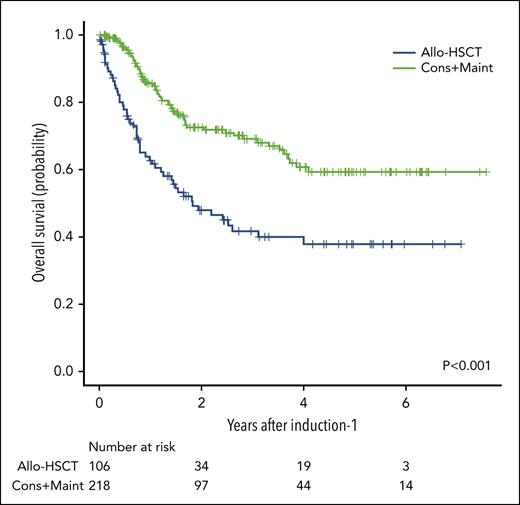
Overall survival and event-free survival for patients from the whole series. (A) Overall survival for the whole series. (B) Event-free survival for the whole series. (C) Overall survival according to the consolidation and maintenance (Cons+Maint) therapy (green line) vs allogeneic hematopoietic stem cell transplantation (allo-HSCT) (blue line) by intention-to treat.
Overall survival and event-free survival for patients from the whole series. (A) Overall survival for the whole series. (B) Event-free survival for the whole series. (C) Overall survival according to the consolidation and maintenance (Cons+Maint) therapy (green line) vs allogeneic hematopoietic stem cell transplantation (allo-HSCT) (blue line) by intention-to treat.
Univariable and multivariable analysis of prognostic factors for OS and CIR
| Variable . | N . | OS univariable HR (95% CI) . | P . | OS multivariable HR (95% CI) . | P . | CIR univariable HR (95% CI) . | P . | CIR multivariable HR (95% CI) . | P . |
|---|---|---|---|---|---|---|---|---|---|
| Age (continuous variable) | 289 | 1.009 (0.992 to 1.027) | .284 | — | 0.980 (0.963 to 0.998) | .029 | NS | ||
| Female sex | 114/289 | 1.135 (0.749 to 1.719) | .550 | — | 1.301 (0.846 to 2.000) | .230 | — | ||
| WBC count (continuous variable) | 289 | 1.002 (1.000 to 1.003) | .017 | 1.003 (1.001 to 1.005) | .002 | 1.003 (1.001 to 1.004) | <.001 | 1.003 (1.001 to 1.004) | <.001 |
| BCP phenotype | 202/289 | 1.604 (0.985 to 2.610) | .057 | NS | 0.934 (0.591 to 1.474 | .770 | — | ||
| HR cytogenetics* | 30/209 | 2.484 (1.409 to 4.380) | .002 | 1.995 (1.109 to 3.587) | .021 | 1.128 (0.504 to 2.528) | .770 | — | |
| MRD ≥0.1% after induction-1 | 66/286 | 1.856 (1.195 to 2.881) | .006 | — | 0.682 (0.388 to 1.198) | .180 | — | ||
| MRD ≥0.01% after induction-1 | 103/282 | 1.684 (1.107 to 2.563) | .015 | 1.641 (1.002 to 2.706) | .049 | 0.850 (0.538 to 1.341) | .480 | — |
| Variable . | N . | OS univariable HR (95% CI) . | P . | OS multivariable HR (95% CI) . | P . | CIR univariable HR (95% CI) . | P . | CIR multivariable HR (95% CI) . | P . |
|---|---|---|---|---|---|---|---|---|---|
| Age (continuous variable) | 289 | 1.009 (0.992 to 1.027) | .284 | — | 0.980 (0.963 to 0.998) | .029 | NS | ||
| Female sex | 114/289 | 1.135 (0.749 to 1.719) | .550 | — | 1.301 (0.846 to 2.000) | .230 | — | ||
| WBC count (continuous variable) | 289 | 1.002 (1.000 to 1.003) | .017 | 1.003 (1.001 to 1.005) | .002 | 1.003 (1.001 to 1.004) | <.001 | 1.003 (1.001 to 1.004) | <.001 |
| BCP phenotype | 202/289 | 1.604 (0.985 to 2.610) | .057 | NS | 0.934 (0.591 to 1.474 | .770 | — | ||
| HR cytogenetics* | 30/209 | 2.484 (1.409 to 4.380) | .002 | 1.995 (1.109 to 3.587) | .021 | 1.128 (0.504 to 2.528) | .770 | — | |
| MRD ≥0.1% after induction-1 | 66/286 | 1.856 (1.195 to 2.881) | .006 | — | 0.682 (0.388 to 1.198) | .180 | — | ||
| MRD ≥0.01% after induction-1 | 103/282 | 1.684 (1.107 to 2.563) | .015 | 1.641 (1.002 to 2.706) | .049 | 0.850 (0.538 to 1.341) | .480 | — |
—, not included in the model for multivariate analysis; HR, hazard ratio; NS, nonsignificant.
HR cytogenetics: t(v;11q23), hypodiploidy, and complex karyotype.
Analysis by ITT
Table 2 shows the demographic and clinical characteristics of patients assigned to chemotherapy and allo-HSCT. The 2 groups were well balanced for most of the clinical and biologic characteristics, except for a higher number of patients with ETP-ALL and with t(v;11q23)/KMT2A rearrangements and a lower number of patients with t(1;19) in the allo-HSCT group (Table 2). Relapse occurred in 74/218 patients assigned to chemotherapy and in 29/90 cases assigned to allo-HSCT, with a 5-year CIR of 45% (37% to 53%) and 40% (28% to 51%), respectively (Figure 3B). Fifty-nine of the 218 patients assigned to chemotherapy and 54/106 assigned to allo-HSCT died. The 5-year OS probabilities were 59% (50% to 68%) and 38% (27% to 49%), respectively (Figure 4C).
Analysis by actual treatment given
The 5-year OS of patients treated in the chemotherapy arm (n = 145) was 72% (61% to 81%); disease progression was the main cause of death. Relapse occurred in 43/145 patients, with a 5-year CIR of 40% (30% to 50%) and a 5-year NRM of 3% (1% to 7%) (supplemental Figure 3A-B). In turn, the 5-year OS probability for 62 transplanted patients was 54% (39% to 67%), with a CIR and NRM of 33% (21% to 47%) and 24% (14% to 37%), respectively (supplemental Figure 3C-D). No differences were observed in major HSCT outcomes (OS, CIR, and NRM) according to the type of donor or to the type of conditioning regimen (data not shown), but the NRM was significantly higher in patients over 50 years of age (5-year incidences: 19% [8% to 34%] vs 43% [17% to 67%], P = .038).
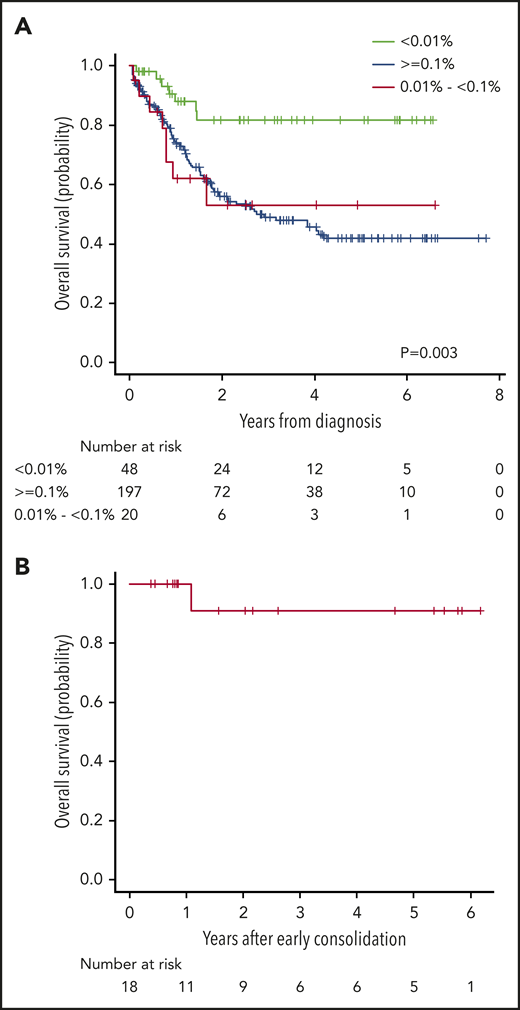
Outcomes of patients with deep early MRD response
The 5-year OS probability of the 48 patients who showed MRD < 0.01% on day 14 of induction was 82% (69% to 95%), which was significantly better than that of patients with an MRD level of 0.01% to <0.1% or those with MRD ≥0.1% (Figure 5A). Eighteen of 48 patients maintained the MRD level <0.01% at the end of induction and at the end of early consolidation. Their demographic and clinical characteristics are depicted in supplemental Table 2 and their 5-year OS probability was 91% (51% to 99%) (Figure 5B).
Overall survival according to the measurable residual disease level. (A) Overall survival according to MRD level on day 14 of induction. Green line denotes survival according to MRD level <0.01%, blue line indicates survival according to MRD level higher than or equal to 0.1% and red line denotes survival according to MRD levels between 0.01% and 0.1%. (B) Overall survival for patients with MRD levels <0.01% on day 14, end of induction, and end of early consolidation.
Overall survival according to the measurable residual disease level. (A) Overall survival according to MRD level on day 14 of induction. Green line denotes survival according to MRD level <0.01%, blue line indicates survival according to MRD level higher than or equal to 0.1% and red line denotes survival according to MRD levels between 0.01% and 0.1%. (B) Overall survival for patients with MRD levels <0.01% on day 14, end of induction, and end of early consolidation.
Toxicity
Selected grade 3 to 5 toxicities in induction-1, in induction-2, and in each early consolidation cycle are summarized in Table 4. As shown, hematologic toxicity and infections were by far the most frequent toxicities, followed by liver toxicity, which was mainly concentrated in induction-1 and was mostly attributed to asparaginase.
Selected grade 3 to 5 toxicities in induction-1, induction-2, and in each cycle of early consolidation
| . | Induction-1 (%) . | Induction-2 (%) . | C-1 (%) . | C-2 (%) . | C-3 (%) . |
|---|---|---|---|---|---|
| Neutropenia | 306/332 (92) | 80/82 (98) | 51/207 (25) | 133/193 (69) | 19/173 (11) |
| Days, median (min; max) | 20 (1; 45) | 19 (3; 60) | 4 (1; 33) | 4 (1; 13) | 4 (1; 10) |
| Thrombocytopenia | 188/326 (58) | 78/82 (95) | 16/206 (8) | 77/193 (40) | 4/173 (2) |
| Days, median (min; max) | 10 (1; 46) | 12 (2; 50) | 2 (1; 33) | 2 (1; 11) | 2 (1; 2) |
| Infection | 175/333 (53)* | 71/84 (85)# | 18/213 (8)†† | 39/198 (20) | 8/180 (4) |
| Hypersensitivity | 3/330 (1) | 0 | 3/209 (1) | 5/198 (3) | 7/179 (4) |
| Neurologic | 19/330 (6)† | 2/82 (2) | 3/211 (1) | 4/199 (2) | 3/180 (2) |
| Hepatic | 88/328 (27)‡ | 7/81 (9) | 15/211 (7) | 4/199 (2) | 9/180 (5) |
| Renal | 4/331 (1) | 0 | 11/211 (5) | 0 | 2/178 (1) |
| Digestive | 12/323 (4)§ | 10/82 (12)** | 3/212 (1) | 1/198 (0.5) | 0 |
| Mucositis | 16/323 (5) | 7/81 (9) | 3/213 (1) | 1/200 (0.5) | 1/179 (1) |
| Coagulation disorders | 35/329 (11) | 0 | 2/208 (1) | 1/196 (0.5) | 0 |
| Vascular | 28/329 (9) | 4/82 (5) | 2/208 (1) | 1/196 (0.5) | 0 |
| Metabolic | 13/329 (4) | 0 | 2/208 (1) | 1/196 (0.5) | 2/176 (1) |
| Cardiac | 3/329 (1)‖ | 0 | 0 | 0 | 0 |
| Hemorrhagic | 2/329 (1)¶ | 0 | 0 | 0 | 0 |
| Endocrine | 1/329 (0.3) | 0 | 1/208 (0.5) | 0 | 0 |
| Musculoskeletal | 3/329 (1) | 0 | 0 | 1/196 (0.5) | 1/176 (1) |
| Psychiatric | 0 | 0 | 0 | 1/196 (0.5) | 0 |
| . | Induction-1 (%) . | Induction-2 (%) . | C-1 (%) . | C-2 (%) . | C-3 (%) . |
|---|---|---|---|---|---|
| Neutropenia | 306/332 (92) | 80/82 (98) | 51/207 (25) | 133/193 (69) | 19/173 (11) |
| Days, median (min; max) | 20 (1; 45) | 19 (3; 60) | 4 (1; 33) | 4 (1; 13) | 4 (1; 10) |
| Thrombocytopenia | 188/326 (58) | 78/82 (95) | 16/206 (8) | 77/193 (40) | 4/173 (2) |
| Days, median (min; max) | 10 (1; 46) | 12 (2; 50) | 2 (1; 33) | 2 (1; 11) | 2 (1; 2) |
| Infection | 175/333 (53)* | 71/84 (85)# | 18/213 (8)†† | 39/198 (20) | 8/180 (4) |
| Hypersensitivity | 3/330 (1) | 0 | 3/209 (1) | 5/198 (3) | 7/179 (4) |
| Neurologic | 19/330 (6)† | 2/82 (2) | 3/211 (1) | 4/199 (2) | 3/180 (2) |
| Hepatic | 88/328 (27)‡ | 7/81 (9) | 15/211 (7) | 4/199 (2) | 9/180 (5) |
| Renal | 4/331 (1) | 0 | 11/211 (5) | 0 | 2/178 (1) |
| Digestive | 12/323 (4)§ | 10/82 (12)** | 3/212 (1) | 1/198 (0.5) | 0 |
| Mucositis | 16/323 (5) | 7/81 (9) | 3/213 (1) | 1/200 (0.5) | 1/179 (1) |
| Coagulation disorders | 35/329 (11) | 0 | 2/208 (1) | 1/196 (0.5) | 0 |
| Vascular | 28/329 (9) | 4/82 (5) | 2/208 (1) | 1/196 (0.5) | 0 |
| Metabolic | 13/329 (4) | 0 | 2/208 (1) | 1/196 (0.5) | 2/176 (1) |
| Cardiac | 3/329 (1)‖ | 0 | 0 | 0 | 0 |
| Hemorrhagic | 2/329 (1)¶ | 0 | 0 | 0 | 0 |
| Endocrine | 1/329 (0.3) | 0 | 1/208 (0.5) | 0 | 0 |
| Musculoskeletal | 3/329 (1) | 0 | 0 | 1/196 (0.5) | 1/176 (1) |
| Psychiatric | 0 | 0 | 0 | 1/196 (0.5) | 0 |
C-1, early consolidation-1; C-2, early consolidation-2; C-3, early consolidation-3.
Eight patients with grade 5 (G5).
One patient with G5.
One patient with G5.
One patient with G5.
One patient with G5.
Two patients with G5.
Nine patients with G5.
One patient with G5.
One patient with G5.
Comparison with the results from the ALL-AR-03 trial
There was a significant improvement in OS and EFS in this trial compared with the ALL-AR-03 trial, even when patients with the same HR features at baseline were compared (5-year OS 49% [42% to 56%] vs 32% [25% to 39%], P < .001 and 5-year EFS 40% [34% to 47%] vs 30% [21% to 36%], P = .003; supplemental Figure 4A-B). The sum of several features may explain the better results observed in the ALL-HR-11 trial. First, a slight but nonsignificant lower death rate in induction-1 (5% vs 8%, P = .137) and a slightly higher CR rate (91% vs 85%, P = .067); second, a significantly lower death rate during consolidation (1% vs 10%, P < .001); and third, a trend for better allo-HSCT outcomes (5-year OS 54% [39% to 67%] vs 39% [23% to 55%], P = .118) because of lower NRM (24% [14% to 37%] vs 37% [21% to 54%], P = .183).
Discussion
This prospective study, restricted to adolescents and adult patients with Ph− ALL with HR features, showed that patients with good early MRD response to induction therapy and complete MRD response after early consolidation treatment have promising outcomes with pediatric-inspired chemotherapy without allo-HSCT. On the other hand, early assignment to allo-HSCT might provide long-term response in a fraction of patients showing suboptimal response to induction-1, defined as failure to achieve an MRD level <0.1%. A small fraction of patients with sustained deep MRD response already from day 14 of first induction cycle onward showed excellent outcomes.
MRD is the most important prognostic factor in both children and adults with ALL,18-21 irrespective of the MRD method (polymerase chain reaction for immunoglobulin/ T-cell receptor rearrangements, FCM, or next-generation sequencing), threshold (MRD <0.01% is defined as MRD response by consensus)22 and the timepoints of measurement used (after induction and/or after consolidation are the most frequently used). Regardless of the risk factors at baseline, in adults with Ph− ALL, several studies have shown that allo-HSCT in first CR is the best option for patients with poor MRD response defined as postinduction levels ≥0.1% and/or postconsolidation levels ≥0.01%.23-25 Consequently, most ongoing trials use this parameter to select the type and intensity of treatment, once morphological CR has been achieved. As there is no doubt that allo-HSCT benefits patients with poor MRD response after induction or consolidation, the indication of allo-HSCT in cases of good MRD clearance is not clear. In this sense, it is important to note that allo-HSCT only benefited MRD+ patients in 2 studies from the GRAALL Group.24,25
The allocation of adult patients with Ph− ALL to chemotherapy or to allo-HSCT as postconsolidation therapy according to MRD levels has been addressed in some prospective trials. The first was conducted by the Northern Italy Study Group (NILG).26 Allocation of chemotherapy vs allo-HSCT was performed ac-cording to MRD response (assessed by immunoglobulin/T-cell receptor rearrangements by quantitative reverse-transcription polymerase chain reaction at weeks 10/16 and 22) and to risk at baseline. The 5-year OS probability was 73% for patients assigned to chemotherapy and 58% for those assigned to HSCT (n = 87, 61%) by ITT. A subsequent study by the NILG used similar criteria for risk definition and chemotherapy assignment, but 2 timepoints were considered for allo-HSCT allocation: (1) early (after week 10) for all patients with very HR ALL or those with SR/HR ALL with MRD ≥0.01% at week 10 or HR with unknown MRD levels; or (2) late (after week 22) for SR/HR cases with MRD ≥0.01% at week 16 or MRD+ at week 22. The 5-year OS probabilities for these 2 groups were 73% and 58%, respectively (P = .078), with a similar 5-year CIR for both groups (35% vs 37%, respectively).27 The GMALL Group is randomly evaluating allo-HSCT vs chemotherapy in patients with HR ALL with MRD response after consolidation (clinicaltrials.gov #NCT02881086).28
A previous trial by our group (ALL-AR-03) used a composite endpoint (standard morphologic response at day 14 of induction therapy and MRD <0.05% after early consolidation) for assignment of postconsolidation therapy in 326 patients with HR, Ph− ALL.15 Apart from the use of the MRD level criteria alone for treatment assignment in the current ALL-HR-11 trial, some differences between the 2 trials should be pointed out. First, the criteria for HR definition were more stringent in the present study. Second, an induction-2 (FLAG-Ida) cycle was given to patients without CR or with poor MRD response, instead of continuing with the first consolidation cycle (as a second induction therapy) for similar patients in the former study. Third, the dose of methotrexate was increased to 5 g/m2 in patients with T-ALL, and pegylated asparaginase was used in some centers in the current trial. Fourth, MRD was centrally assessed with a high-sensitivity 8-color FCM approach as demonstrated by the EuroFlow group (2 × 10−6 vs 1 × 10−4),17 and the postearly consolidation MRD level chosen for assignment to chemotherapy was stricter (0.01% vs 0.05%). Apart from the possible impact of these modifications, the main reason for the better outcomes observed in the current trial was a reduction of treatment-related deaths in both the chemotherapy and the transplant arms. The reduction of the weekly daunorubicin dose from 60 mg/m2 to 45 mg/m2 in induction and, especially, the elimination of some myeloablative and marginally active drugs given in consolidation (mitoxantrone, cyclophosphamide, and etoposide) in the ALL-HR-11 trial compared with the ALL-AR-03 trial could have contributed to the highly significant reduction of mortality during consolidation. The use of PEG-asparaginase in this trial according to center availability did not contribute to improving the results,29-31 as was demonstrated in an interim analysis showing nonsignificant differences in CR, MRD levels after induction and after consolidation, disease-free survival, and OS.32 In addition, no significant differences in grades 3 to 4 toxicity were observed in the induction period, although a trend to higher hepatic toxicity was observed in patients receiving PEG-aparaginase.32
Despite being an exploratory endpoint, 68/265 patients (26%) showed MRD levels <0.1% on day 14 of induction therapy, and in 48 the MRD levels were <0.01% (18%). These patients showed a better OS than the remaining patients. Eighteen of these 48 patients maintained this low MRD level until the postearly consolidation assessment, and their outcomes were very promising. Although this group was small, our results indicate that early and sustained MRD clearance could be predictive of good outcomes (as occurs in pediatric trials),29 even in adult patients with HR features. In turn, it is important to note that progressively decreased MRD levels throughout therapy were observed in our study, with MRD < 0.01% in 91% of patients who completed early consolidation therapy.
A high WBC count, HR cytogenetics, and an MRD level ≥0.01% after induction were the 3 independent poor prognostic factors for OS identified in our study. WBC counts and MRD levels have consistently been identified in several prospective trials.15,22,23,26,27 The end-induction MRD level <0.01% with prognostic significance in our study would suggest that in future studies this level should be considered for postinduction assignation of treatment instead of 0.1%.33 The identification of HR cytogenetics as an independent variable for OS in the present trial points to the need for considering these patients as very-HR patients, for whom improved induction/consolidation therapy followed by early allo-HSCT or other cellular immunotherapies is needed. In some adult ALL trials (considering patients with SR and HR ALL together), patients with T-ALL have shown better outcomes than those with BCP-ALL.27,30,31 Despite the dose of methotrexate being increased in T-ALL compared with BCP-ALL patients in our study, no differences in outcome were observed in HR patients with T vs BCP-ALL, although patients with ETP-ALL had poor outcomes compared with the remaining T-ALL cases.16 The exclusive inclusion of poor-risk T-ALL patients in our trial could explain this lack of differences with BCP-ALL. Regarding the CIR, a high WBC count was the only independent poor prognostic factor identified by multivariable analysis. The unfavorable prognostic significance of younger age shown in univariable analysis can be explained by the fact that patients younger than 30 years had more disease-related HR features (especially high WBC count) than older patients. Older-aged patients without any other risk factor could enter the trial contrary to younger patients, who required additional HR features to be evaluable for inclusion.
Although MRD is considered the strongest risk factor in ALL, its predictive value for relapse assessment is limited and was not identified as an independent variable for CIR in our trial. This poor predictive value of MRD was confirmed in a study by the GRAALL Group,30 in which the CIR was 21% in patients with an end-induction MRD level <0.01%, irrespective of censoring or not the follow-up of patients at the time of HSCT. In the 09/00 study by the NILG, the CIR according to treatment allocation based on MRD evaluation at week 10 was similar (5-year probability of 35% in patients assigned to maintenance chemotherapy and of 37% for those assigned to allo-HSCT).27 Our study showed similar results (5-year CIR of 45% for patients allocated to chemotherapy and of 40% for those assigned to allo-HSCT), with the higher incidence being attributable to the sole enrollment of patients with HR features at baseline in our study. Of note, the incidence of relapse was 33% in patients submitted to allo-HSCT vs 40% in patients that received chemotherapy, despite the transplanted group, including patients with more resistant and poor-risk leukemia (eg, higher frequency of cases with early pre-T-ALL or ALL with t(v;11q23) rearrangements), indicating the ability of allo-HSCT to rescue at least some HR patients. Unfortunately, the 24% cumulative incidence of NRM counterbalanced this antileukemic activity and contributed to explaining the poorer results in transplanted patients. However, the high CIR, despite good MRD clearance, points to the need for exploring ways to improve the outcomes of these patients (eg, use of more sensitive methods of MRD detection or lower cutoff values of MRD for therapy assignment, or inclusion of more effective postconsolidation/maintenance therapies, such as immunotherapy and/or targeted therapies).
Although this was a prospective study, including a large number of adolescents and adults with Ph− ALL selected according to widely recognized HR features, in which MRD was centrally assessed by an optimized FCM technique used as the only parameter for treatment allocation, some limitations should be pointed out. First, there was a significant proportion of protocol deviations (14% in the group assigned to chemotherapy and 13% to the patients assigned to allo-HSCT by ITT), particularly among patients with end-consolidation MRD ≥ 0.01% (10/15 patients continued with chemotherapy instead of being submitted to allo-HSCT as indicated per protocol). Most deviations were due to physician or patient decision, and some were related to the approval of immunotherapy in earlier phases of ALL (3 patients received blinatumomab after approval of this drug for use in CR patients with MRD+ status) or with the inclusion of patients in clinical trials (6 received blinatumomab as part of consolidation in a phase 2 clinical trial by our group (clinicaltrials.gov #NCT03523429).32 These patients were censored for follow-up at the initiation of blinatumomab therapy. The second limitation refers to the use of native or pegylated E coli asparaginase according to center availability because the doses of both formulations were not bioequivalent in the consolidation cycles. However, the outcomes and toxicity did not significantly differ according to the type of asparaginase given.32 The third limitation was the sole focus on MRD levels to assign therapy. Some studies have shown that genetic features of ALL have prognostic significance independently of the MRD, with differences for BCP-ALL and for T-ALL.30 Our study showed that HR cytogenetics also had independent prognostic significance. Other studies have pointed out the prognostic significance of secondary genetic abnormalities in both children and adults with ALL.34-36 In addition, a study from the pediatric UKALL 2003 trial showed that the absolute relapse rate associated with a specific MRD value varied significantly according to the genetic subtype of ALL.37 Thus, integration of genetic subtype/subclone-specific MRD might potentially allow for more refined risk stratification. Finally, the BCR-ABL-like ALL subtype, associated with poor prognosis in both children and adults,38 was not identified in our cohort.
Our study focused only on the use of standard chemotherapy and/or allo-HSCT. However, in recent years, significant improvements have been achieved from the experience gained with the use of improved targeted therapy and immunotherapy (with monoclonal antibodies or cell-based therapies) in patients with refractory or relapsed ALL.7,39-42 Some of these improvements have been successfully used in CR patients with positive MRD and even in patients newly diagnosed with ALL.43,44 Preliminary experiences have recently shown that the combination of targeted therapies and immunotherapy allowed a reduction or virtual elimination of chemotherapy in some ALL subtypes, such as Ph+ ALL. It is highly probable that the incorporation of these new strategies in frontline ALL therapy, combined with genetic and MRD-based stratification of therapy, will contribute to improving the outcomes of adult patients with ALL. Finally, the possible use of lower cutoff values of end-induction or end-consolidation MRD for treatment decisions could improve the selection of patients for the consolidation therapies available leading to potentially lower CIR rates and improved survival probabilities.
Presented as oral session at the 61st Annual Meeting of the American Society of Hematology, Orlando, FL, 7-10 December 2019.
For original data, please contact jribera@iconcologia.net or mmorgades@iconcologia.net. Deidentified individual participant data are available indefinitely at www.pethema.org. The study protocol is also available at the same Web site.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Trinquand (Assistance Publique–Hôpitaux de Paris, Paris, France), T. Kalina (DPH/O, Prague, Czech Republic), M. Brüggemann (University Medical Center Schleswig-Holstein [UKSH], Kiel, Germany), M. Ritgen (UKSH), V. H. J. van der Velden (Erasmus MC, Rotterdam, The Netherlands), T. Szczepanski (Zabrze, Poland), L. Sędek (Zabrze, Poland), J. Philippé (Ghent, Belgium), A. van der Sluis (Leiden University Medical Center [LUMC], Leiden, The Netherlands), J. Flores-Montero (University of Salamanca [USAL], Salamanca, Spain), S. Barrena (USAL), and J. J. M. van Dongen (LUMC), and all other members of EuroFlow for their contribution in the design and validation of the EuroFlow MRD panels under the support of the EuroFlow FP6-2004-LIFESCIHEALTH5 program of the European Commission (grant LSHB-CT-2006-018708) as Specific Targeted Research Project (STREP).
This project was supported by the Instituto de Salud Carlos III (ISCIII) (PI14/01971 and PI19/01828), cofunded by European Regional Development Fund (ERDF)/European Social Fund (ESF), “A way to make Europe”/“Investing in your future,” Centres de Recerca de Catalunya (CERCA)/Generalitat de Catalunya SGR 2017 288 (GRC)/“La Caixa.”
The following Spanish institutions participated in the study: Institut Català d’Oncologia-Hospital Germans Trias i Pujol, Josep Carreras Research Institute, Badalona, Universitat Autònoma de Barcelona, Spain; IBSAL, IBMCC, Centro de Investigación del Cáncer, Universidad de Salamanca-CSIC, Hospital Universitario, Salamanca, Spain; Hospital Universitario La Fe, Valencia, Spain; Hospital Clínic, Barcelona, Spain; Hospital Vall d’Hebron, Barcelona, Spain; Hospital de Sant Pau, Barcelona, Spain; Hospital Virgen de la Victoria, Málaga, Spain; Hospital Central de Asturias, Oviedo, Spain; Hospital Doce de Octubre, Madrid, Spain; Hospital Gregorio Marañón, Madrid, Spain; Hospital General, Alicante, Spain; Hospital Clínico, Valencia, Spain; Hospital Universitario, Donostia, Spain; Institut Català d’Oncologia-Hospital Joan XXIII, Tarragona, Spain; Hospital Universitario Virgen del Rocío, Sevilla, Spain; Hospital Universitario Doctor Negrín, Las Palmas de Gran Canaria, Spain; Hospital Marqués de Valdecilla, Santander, Spain; Hospital Son Espases, Palma de Mallorca, Spain; Hospital Universitario de Canarias, Santa Cruz de Tenerife, Spain; Institut Català d’Oncologia-Hospital Josep Trueta, Girona, Spain; Hospital Morales Meseguer, Murcia, Spain; Hospital Arnau de Vilanova, Valencia, Spain; Hospital Universitario Insular, Las Palmas de Gran Canaria, Spain; Hospital Reina Sofia, Córdoba, Spain; Institut Català d’Oncologia-Hospital Duran i Reynals, L’Hospitalet de Llobregat, Spain; Hospital Son Llàtzer, Palma de Mallorca, Spain; Hospital Italiano, Buenos Aires, Argentina; Hospital Clínico, Valladolid, Spain; Hospital del Mar, Barcelona, Spain; Hospital Universitari Mútua, Terrassa, Spain; Hospital Arnau de Vilanova, Lleida, Spain; Hospital San Pedro de Alcántara, Cáceres, Spain; Hospital Miguel Servet, Zaragoza, Spain; Hospital Lucus Augusti, Lugo, Spain; Hospital HM Sanchinarro, Madrid, Spain; Hospital Ramón y Cajal, Madrid, Spain; Hospital Clínico, Santiago de Compostela, Spain; Complejo Hospitalario, Ourense, Spain; Hospital General, Castellón, Spain; Hospital Universitario, Galdakao, Spain; Hospital La Zarzuela, Madrid, Spain; Complejo Hospitalario, Pontevedra, Spain; Hospital Verge de la Cinta, Tortosa, Spain; Hospital General, Albacete, Spain; Hospital Río Carrión, Palencia, Spain; Hospital Virgen de la Arrixaca, Murcia, Spain; Hospital de Valme, Sevilla, Spain; Hospital Fuenlabrada, Madrid, Spain; Hospital Infanta Sofía, Madrid, Spain; Hospital Doctor Peset, Valencia, Spain; Complejo Hospitalario, Navarra, Spain; Complejo Hospitalario, Jaén, Spain; Hospital de Basurto, Bilbao, Spain.
Authorship
Contribution: J.-M.R. conceived and designed the trial, contributed to the analysis of the results, and wrote the paper; M.M. collected data, created the database, and performed the statistical analysis; J.C., S.B., L.L., and A.O. performed the centralized MRD analysis; E.G., J.R., and I.G. reviewed the charts from cytogenetic and immunophenotypic studies at diagnosis; J.-M.R., M.M., J.E., P.B., and A.O. contributed to the analysis and interpretation of the data; P.M., I.G.-C., M.J.M., D.M.-C., A.T., P.M.-S., S.M., C.G., M.T., M.T.A., M. Cervera, J.G.-C., C.R., A.B., A.N., B.S., R.C., M.-L.A., A.L.-M., R.F.-M., J.S., S.M., A.C., A.G.-C., M.-J.P., E.A., F.V.-l., J.-M.H.-R., A.G.-G., J.-M.B., B.d.R., M.-J.S.-S., A.S., M. Calbacho, N.A., J.-A.M.-S., R.G.-B., M.O., L.Z., I.G., and E.F. reported the patients and followed them clinically; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: J.-M.R. receives consultancy fees, research funding, and speakers bureau fees from Pfizer and Amgen; consultancy and speakers bureau fees from Ariad and Novartis. P.M. serves as membership on an entity’s board of directors or advisory committees for Abbvie, serves as consultancy, membership on an entity’s board of directors or advisory committees, other: research support and speakers bureau for Celgene and Daiichi Sankyo, membership on an entity’s board of directors or advisory committees and speakers bureau for Incyte, serves on membership on an entity’s board of directors or advisory committees, other: receives research support, research funding, and speakers bureau for Janssen; serves as membership on an entity’s board of directors or advisory committees and other: receives research support for Karyopharm; serves on membership on an entity’s board of directors or advisory committees, other: receives research support, research funding, and speakers bureau fees for Novartis, Pfizer, Teva, serves on membership on an entity’s board of directors or advisory committees for Jazz Pharmaceutical. J.E. serves as consultancy for or receives travel grants from Amgen, Roche and serves as consultancy for Pfizer and Jazz Pharmaceuticals. P.B. serves as consultancy and as a speaker for Amgen, Celgene, Pfizer, serves as consultancy for Gilead, Jazz Pharmaceuticals, Novartis, and Shire. M.T. serves as consultancy and has received fees from sponsored conferences and meetings for Celgene, serves as consultancy and sponsored conferences and meetings for Novartis, serves as consultancy for Astellas, has sponsored conferences and meetings for Pfizer and Servier. A.B. serves as consultancy, serves on membership on an entity’s board of directors or advisory committees and speakers bureau for Celgene Corporation, serves as consultancy and on membership on an entity’s board of directors or advisory committees for Amgen and Fresenius, serves on membership on an entity’s board of directors or advisory committees for Janssen, serves as consultancy and on speakers bureau for MSD. M.-J.P. serves on the advisory board for Novartis, Amgen, Incyte, serves on the advisory board and as speaker for Celgene, and serves as a speaker for Abbvie, Takeda, and Roche. A.O. and L.L. report being inventors on the EuroFlow-owned patent PCT WO 2013/187765A2 (Methods, reagents, and kits for detecting minimal residual disease). The Infinicyt software is based on intellectual property of the EuroFlow laboratories (University of Salamanca and Federal University of Rio de Janeiro, Rio de Janeiro, Brazil) and the scientific input of other EuroFlow members. All the above-mentioned intellectual property and related patents are licensed to Cytognos (Salamanca, Spain) and BD Biosciences (San José, CA), which pay royalties to the EuroFlow Consortium. These royalties are exclusively used for continuation of the EuroFlow collaboration and sustainability of the EuroFlow consortium. The remaining authors declare no competing financial interests.
Correspondence: Josep-Maria Ribera, Clinical Hematology Department, ICO-Hospital Germans Trias i Pujol, C/Canyet s/n, 08916 Badalona, Spain; e-mail: jribera@iconcologia.net.


Comments
Typo error in previous comment
I believe the axes in the Visual abstract figure are reversed ?