In this issue of Blood, Pleyer and colleagues report results from 2 studies assessing differences in the humoral response to 2 different vaccines in patients with chronic lymphocytic leukemia (CLL) on observation or receiving a Bruton tyrosine kinase inhibitor (BTKi).1 Their findings have immediate clinical implications and call for research preparedness as we eagerly anticipate access to vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the near future.
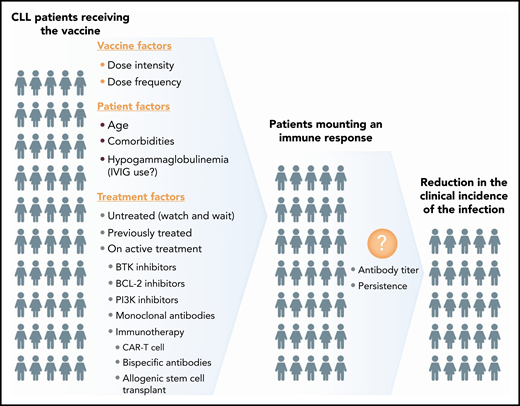
Variables to consider when studying the efficacy of COVID-19 vaccine in patients with CLL. IVIG, intravenous immunoglobulin.
Variables to consider when studying the efficacy of COVID-19 vaccine in patients with CLL. IVIG, intravenous immunoglobulin.
Despite recent advancements in the treatment of CLL, our understanding of the potential impact of novel agents on the immune response to vaccinations is limited. Suboptimal humoral response to vaccination has been reported in CLL.2 In recent years, novel agents, namely inhibitors of BTK, phosphoinositide 3-kinases, or the antiapoptotic protein, B-cell lymphoma-2, have changed the treatment landscape for CLL.3 A growing proportion of patients now have indefinite or long-term exposure to these drugs that directly affect the immune system, potentially further dampening their ability to mount the appropriate response to vaccinations. Seroconversion after the seasonal influenza vaccine in patients receiving ibrutinib has been reported to be as low as 7% in 1 study evaluating the standard-dose vaccine and 26% in another in which a proportion of patients received a higher dose.4,5 Given these alarming numbers, this remains an important area for investigation for the CLL community.
Pleyer and colleagues from the National Heart, Lung, and Blood Institute evaluated serologic responses with the adjuvanted recombinant hepatitis B (HepB-CpG) and zoster (RZV) vaccines in patients with treatment-naive (TN) CLL and those receiving BTKi’s. Seroconversion was measured 6 months after vaccination. De novo immune response was assessed in the HepB-CpG study; investigators observed a significant difference in antibody response between the TN (28%) and BTKi (3.8%) cohorts. In contrast, when assessing for recall antibody response with the RZV vaccine, there was no difference in serologic response between the 2 cohorts (59% vs 41%). Given the lack of de novo humoral response to the HepB-CpG vaccine in the BTKi cohort, the authors appropriately suggested that vaccination against novel antigens may need to be considered well before initiating the BTKi therapy.1
The finding of comparable serologic responses to the RZV in patients receiving BTKi therapy is promising and confirms current recommendations. Notably, another recent study by Zent et al also showed a high rate of early (1 month) humoral and cellular responses in patients with CLL and lymphoplasmacytic lymphoma receiving BTKi’s.6 Together, these studies provide a strong basis for larger confirmatory trials to better inform practitioners regarding appropriate vaccination strategies for patients with CLL and other lymphoid malignancies. In the meantime, these data can be used to support the use of RZV vaccine for CLL patients on a BTKi. Given the various indications for first- (ibrutinib) and second- (acalabrutinib, zanubrutinib) generation BTKi’s in lymphoid malignancies, this could have broader clinical implications.
Lack of serologic response to the HepB-CPG vaccine in BTKi-treated patients is concerning not only for HepB prevention but also in regard to any vaccine designed against other novel antigens as well. The most relevant and prime examples of such vaccines are those for SARS-CoV-2. Although the COVID-19 global pandemic continues to be the leading public health issue, preliminary data indicating the efficacy of messenger RNA–based vaccines in immunocompetent patients have been promising.7,8 However, an important and unanswered question is the efficacy of those vaccines in patients with an impaired immune state because of their underlying condition or/and CLL-specific therapies. In fact, the development of an adequate serologic response after SARS-CoV-2 infection is compromised in CLL, with only one-third of patients developing detectable immunoglobulin G antibodies after a median of ∼2 months after infection, based on 1 study.9
Therefore, while we await the US Food and Drug Administration’s approval of a SARS-CoV-2 vaccine(s), it is imperative to design studies to assess their efficacy in patients with lymphoid malignancies, including CLL. Such studies should be planned early to assure inclusiveness, as many patients are expected to receive the vaccine as soon as it becomes available. The CLL research community has already developed a COVID-19/CLL consortium and presented inferior outcomes in this population.10 Ideally, we will extend these efforts to a comprehensive vaccine database that will allow for uniform data collection promptly. In order to be clinically informative, such a database should include (1) patient characteristics, (2) specifics of the vaccine(s) (type, intensity, frequency), and (3) anti-CLL therapy (see figure). The main emphasis should focus on the impact of CLL-specific treatments. Will “watch-and-wait” patients have a different response to the vaccine? Will there be a meaningful difference in seroconversion in patients receiving BTKi vs venetoclax? Should patients be strategically vaccinated prior to initiation of therapy, and if so, how much earlier? In patients with stable disease, is it reasonable to hold the CLL treatment temporarily to allow for an antibody response to the vaccine? If so, what is a reasonable duration for holding? Is there a significant advantage (or disadvantage) of a time-limited therapy before vaccination? What is the impact of previous treatment with monoclonal antibodies or cellular therapy approaches (allogeneic hematopoietic transplant or chimeric antigen receptor T-cell therapy)? More importantly, although timing and quality of a serologic response as a surrogate endpoint are critical, the main question is to understand the clinical impact of vaccination, including level of risk reduction for SARS-CoV-2 infection and identification of possible predictors of such immune response in patients with CLL.
Although these questions should ideally be addressed in the setting of clinical trials, in the absence of such studies in the foreseeable future, the real-world evidence (RWE) platform seems to be a reasonable approach to answer some of these important practical questions. Given the successful experience of the CLL research community in collaborative efforts and utilizing the RWE in clinical practice, similar collaborations to answer these timely questions are expected in the near future.10
Conflict-of-interest disclosure: M.S. has received consultancy fees, served on advisory boards, steering committees, or data safety monitoring committees for Abbvie, Genentech, AstraZeneca, Sound Biologics, Pharmacyclics, Verastem, ADC Therapeutics, Beigene, Cellectar, Bristol Myers Squibb, Morphosys, TG Therapeutics, Innate Pharma, and Atara Biotherapeutics, and has received research funding from Mustang Bio, Celgene, Bristol Myers Squibb, Pharmacyclics, Gilead, Genentech, Abbvie, TG Therapeutics, Beigene, AstraZeneca, Sunesis, and Beigene. C.U. has received consultancy fees from Abbvie, Pharmacyclics, AstraZeneca, Gilead/Kite Pharma, Morphosys, Verastem, TG Therapeutics, Epizyme, and Atara and has received research funding from Abbvie, Pharmacyclics, AstraZeneca, Gilead/Kite Pharma, and Loxo Oncology.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal