In this issue of Blood, Collins et al have developed a novel multicolor flow cytometry technology to reveal a crucial role of myeloid-derived suppressor cells in Epstein-Barr virus (EBV)–associated lymphoproliferative diseases. In particular this study focuses on EBV-positive natural killer (NK) cell and T-cell proliferation.1
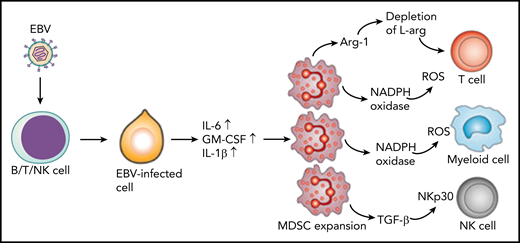
Latent membrane protein-1 (LMP1) in EBV-infected cells induces increased expression of IL-6, GM-CSF, and IL-1β through increased glycolysis. These cytokines promote expansion of MDSCs that suppress T cells, NK cells, and other myeloid cells through different mechanisms. T cells are suppressed through the depletion of L-arginine (L-arg) or via the production of reactive oxygen species (ROS). MDSCs inhibit NK cells through expression of transforming growth factor β (TGF-β) and downregulation of NK-cell–activating receptor NKp30. In addition, MDSCs suppress differentiation of myeloid cells through the ROS-dependent pathway. Arg-1, arginase-1; NADPH, nicotinamide adenine dinucleotide phosphate hydrogen.
Latent membrane protein-1 (LMP1) in EBV-infected cells induces increased expression of IL-6, GM-CSF, and IL-1β through increased glycolysis. These cytokines promote expansion of MDSCs that suppress T cells, NK cells, and other myeloid cells through different mechanisms. T cells are suppressed through the depletion of L-arginine (L-arg) or via the production of reactive oxygen species (ROS). MDSCs inhibit NK cells through expression of transforming growth factor β (TGF-β) and downregulation of NK-cell–activating receptor NKp30. In addition, MDSCs suppress differentiation of myeloid cells through the ROS-dependent pathway. Arg-1, arginase-1; NADPH, nicotinamide adenine dinucleotide phosphate hydrogen.
Primary EBV infection is often acquired in early childhood with minimal clinical consequences. Soon after infection, EBV establishes a latent infection in B cells and has successfully evolved to exploit cellular transcriptional machinery to avoid immune recognition by CD8+ and CD4+ T cells. However, when adolescents acquire primary EBV infection, it can lead to a severe immune reaction, which can manifest as infectious mononucleosis. On rare occasions, this primary exposure can progress to a chronic, nonresolving condition termed chronic active EBV (CAEBV). Patients with this disease present with clinical features such as fever, splenomegaly, hepatitis, and lymphadenopathy, similar to symptoms observed in infectious mononucleosis but at a much more significant level.2 These symptoms can last for 3 months during which a high load of EBV DNA is observed in the blood with concurrent heavy infiltration of EBV-infected cells in organs. Notably, in patients with indolent disease, the disease can remain stable for years, but in some cases, CAEBV might progress to potentially fatal conditions such as hepatic failure and gastrointestinal ulceration or perforation with hemophagocytic lymphohistiocytosis (HLH).2 The prognosis for patients with CAEBV varies among countries. Western countries have reported that the presence of EBV infection in B cells along with defective cytotoxic T cells or NK cells can be associated with the chronic infection. In contrast, Central and South American and Southeast Asian countries have described the presence of EBV infection in T cells (a more aggressive disease with poor survival) and NK cells (milder symptoms with favorable outcome).2 Notably, NK cell–derived CAEBV has a high potential to transform into much more aggressive disorders such as NK cell leukemia or extra-nodal NK/T-cell lymphoma.
Previous studies on the pathogenesis of B-cell–associated CAEBV have revealed impaired cellular immunity that includes inactivating mutations in effector molecules. However, patients with NK/T-cell–associated CAEBV do not have preexisting immune impairment, which suggests that a more complex pathogenesis may be operating in this clinical setting. Collins et al hypothesized that an inhibitory mechanism may be at play. To test this hypothesis, they developed a highly innovative strategy based on multicolor flow cytometry, which allowed phenotypic and functional profiling with in situ hybridization for the EBV-encoded RNA (EBER) expressed in EBV-infected cells in blood from patients with CAEBV. Five patients who were diagnosed with CAEBV or HLH were included in their study. By using a combination of multicolor flow cytometry and Flow RNA (for EBER staining), the authors were able to precisely identify, enumerate, and phenotypically characterize lymphocyte subsets infected with EBV. Their analysis indicated that 2 patients had B-cell–associated CAEBV; another 3 patients had T-cell– or NK-cell–associated CAEBV. EBV infection of T cells was confined to memory cells (CCR7–CD45RA–), which expressed high levels of CD38 and Ki67, indicating increased activation and proliferation of virus-infected cells. These T cells also expressed multiple cytokines, including interleukin-17A (IL-17A), interferon-γ (INF-γ), and/or tumor necrosis factor (TNF). Furthermore, analysis of T-cell receptor usage revealed predominantly monoclonal expansions of EBV-infected T cells.
One of the most exciting observations of the study by Collins et al was the expansion of myeloid-derived suppressor cells (MDSCs) in the blood of CAEBV patients. These cells expressed CD11b, CD15, CD16, and CD33 but were negative for CD14 and HLA class II, and they outnumbered lymphocytes in 4 of the 5 patients. These cells showed the nuclear morphology of cells undergoing granulopoiesis and were confirmed as MDSCs of granulocyte lineage. Luminex analysis of plasma samples from patients revealed the presence of high concentrations of granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-1β, IL-6, and TNF as well as IL-4, IFN-γ, and IL-10, which may promote the expansion and immunosuppressive activity of MDSCs. Indeed, previous studies by Cai et al3 have demonstrated that EBV-encoded LMP1 protein–mediated glycolysis regulates the expression of GM-CSF, IL-1β, and IL-6 through the NLRP3 inflammasome and enhances the expansion of MDSCs in nasopharyngeal carcinoma (see figure). More importantly, the authors also observed an increased concentration of arginase-1 in patient plasma. Previous studies have shown that arginase-1 expressed by MDSCs leads to depletion of L-arginine (see figure), which is crucial for T-cell effector function and survival.4 Finally, the authors showed that MDSCs from patients with CAEBV inhibited T-cell expansion when compared with MDSCs from healthy individuals. These observations are consistent with previously published data by Zang et al,5 who showed that MDSCs from patients with extranodal NK/T-cell lymphoma inhibit anti-CD3–induced proliferation of CD4+ T cells. Furthermore, MDSCs are independent predictors of overall survival and disease-free survival.5
Another important aspect of the study by Collins et al is the clinical management of patients with EBV-infected NK/T cells. As a standard protocol, dexamethasone or methylprednisolone is used for the treating EBV-associated HLH and CAEBV, because these steroids suppress the transcription of T-cell effector cytokines,6 although they might also cause the loss of circulating virus-specific effector T cells. The authors argue that this loss of effector cells may allow the expansion of infected cells after treatment is completed. Indeed, all evaluable patients showed an increase in the number of EBV-infected cells, whereas white blood cell and lymphocyte counts remained within the normal range. Thus, the restricted use of corticosteroids might counterintuitively exacerbate the underlying disease, leading to poor survival and probable relapse in the long term.
Can we develop improved targeted strategies to eliminate MDSCs without compromising antiviral immunity? Indeed there have been a number of strategies proposed to counter or limit the immunosuppressive effect of MDSCs. These include tumor vaccines that alter the phenotype of MDSCs to pro-inflammatory cells,7 and the use of COX-2 inhibitors,8 all-trans retinoic acid,9 or CpG oligonucelotides.10 Future studies focused on a larger cohort of patients and with more detailed characterization of MSDCs in EBV-associated CAEBV and HLH will help to delineate their role in disease pathogenesis and also provide opportunities to test immunomodulatory agents.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal