TO THE EDITOR:
Recently, Evens et al1 reported on real-world outcomes among 641 newly diagnosed patients with Burkitt lymphoma (BL) between 2009 and 2018. Importantly, they confirmed the benefit of incorporating rituximab to chemotherapy backbones, as was demonstrated in a randomized controlled trial (RCT),2 thereby supporting the value of rituximab in general clinical practice. It is also noteworthy that the authors propose a novel prognostic model to predict survival.
Although we appreciate the study of Evens et al, which add considerably to the scarce literature available on real-world outcomes of BL patients, the generalizability of their study findings might be limited. This concern relates to the following factors: 88% of their study population was managed in an academic center, and the median age was 47 years, with only 24% of patients aged ≥60 years. Also, the overall survival (OS) rates were not corrected for the life expectancy of comparable groups from the general population (ie, relative survival [RS]). Therefore, we present the results of a nationwide, population-based study among 990 adult BL patients diagnosed in The Netherlands between 1989 and 2018, including more older patients and more patients treated in a nonacademic setting compared with the study of Evens et al. Moreover, RS rates instead of OS rates are presented.
Founded in 1989, The Netherlands Cancer Registry (NCR) has an overall coverage of >95% of all newly diagnosed malignancies in The Netherlands.3 We selected all adult (≥18 years) BL patients diagnosed between 1989 and 2018, with survival follow-up through 1 January 2020 from the NCR using International Classification of Diseases for Oncology morphology codes 9687 and 9826. Data on sex, dates of birth and diagnosis, hospital of diagnosis, stage, morphology, vital statistics (ie, alive, death, or emigration), and primary treatment were available in the NCR. Data on rituximab were registered in the NCR for patients diagnosed from 2007. From 2014 onward, data on the specific therapeutic regimen and the hospital of treatment were standardly registered, of which the latter may differ from the diagnostic hospital because of referral practices. The privacy review board of the NCR approved the use of anonymous data for this study, which was conducted in accordance with the Declaration of Helsinki.
RS was calculated to estimate disease-specific survival as the ratio of the patients’ OS to the expected survival of an age-, sex-, and calendar year–matched group from the general population.4,5 The expected survival was calculated from Dutch population life tables using the Ederer II methodology.6 RS was computed according to the epoch of diagnosis (ie, 1989-2002 [prerituximab era], 2003-2008 [introduction of rituximab], and 2009-2018 [rituximab as the standard of care]) and age at diagnosis (ie, 18-39, 40-59, 60-75, and >75 years) and measured from diagnosis to death, emigration, or end of follow-up, whichever occurred first. The latter epoch is congruent with the epoch of Evens et al. In contrast to Evens et al, we specifically analyzed patients >75 years, because these patients usually are not treated with high-dose regimens. Multivariable Poisson regression was used to assess linear trends in RS between the first and last epoch and the relative excess risk of mortality. A value of P < .05 indicates statistical significance. Further details about the methods are presented in the supplemental Appendix, available on the Blood Web site.
Our analytical cohort included 990 adult BL patients (median age, 53 years; 40% aged ≥60 years; 14% >75 years, 69% males; and 69% stage III/IV disease; supplemental Table 1A), of whom 39% were diagnosed in an academic hospital. Additional risk factors such as elevated lactate dehydrogenase, extranodal site involvement, and central nervous system involvement were available from 2014 (supplemental Table 1B).
The overall age-standardized incidence rate in the most recent epoch was 3.0 per 1 000 000 person-years and varied according to age and stage (supplemental Table 2; supplemental Figure 1).
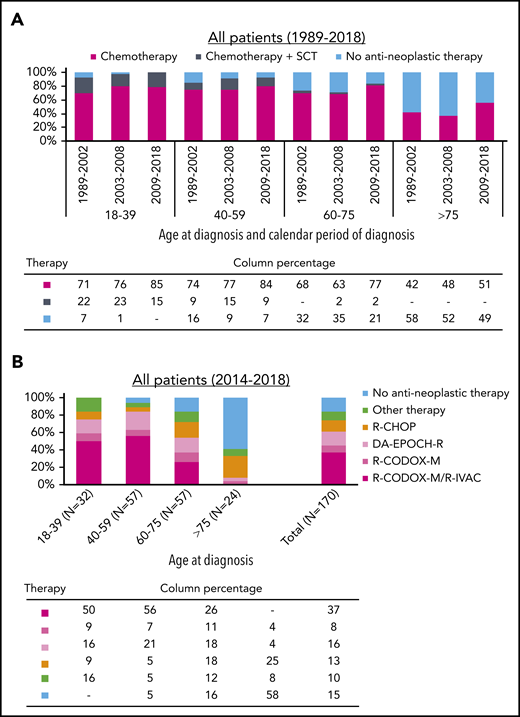
As presented in Figure 1A and supplemental Table 3, chemotherapeutic treatment increased with each successive epoch across all age groups. Nevertheless, the application of chemotherapy was lower in patients aged >75 years compared with those aged 18 to 75 years. Virtually all patients aged 18 to 39 years received chemotherapy from 2003. Of note, the application of stem cell transplantation was mostly part of a clinical trial.7 Last, 87% of patients received rituximab-containing chemotherapy from 2009 to 2018 (supplemental Table 4).
Primary therapy of adult patients with Burkitt lymphoma in The Netherlands. (A) Application of primary therapy among 990 patients diagnosed between 1989 and 2018 according to the 3 broadly defined categories of primary therapy. The absolute number of patients within a specific calendar period and age group in panel A is shown in supplemental Table 3. The proportion of rituximab added to chemotherapy among patients diagnosed during 2009 to 2018 is shown in supplemental Table 4 according to age at diagnosis. (B) Application of primary therapy among 170 patients (median age, 59 years; interquartile age range, 44-70 years; 79% males; and 87% stage III-IV) diagnosed between 2014 and 2018 according to the exact therapeutic regimen, stratified by age at diagnosis. The absolute number of patients within a specific age and treatment group in panel B is shown in supplemental Table 5. Of note, 17 (10%) of 170 patients diagnosed between 2014 and 2018 received a variety of regimens. These exact regimens are presented in supplemental Table 6. DA, dose-adjusted; EPOCH-R, rituximab with etoposide, vincristine, and doxorubicin; R-IVAC, rituximab, ifosfamide, etoposide, and high-dose cytarabine; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CODOX-M, rituximab, cyclophosphamide, doxorubicin, vincristine, and methotrexate; SCT, stem cell transplantation.
Primary therapy of adult patients with Burkitt lymphoma in The Netherlands. (A) Application of primary therapy among 990 patients diagnosed between 1989 and 2018 according to the 3 broadly defined categories of primary therapy. The absolute number of patients within a specific calendar period and age group in panel A is shown in supplemental Table 3. The proportion of rituximab added to chemotherapy among patients diagnosed during 2009 to 2018 is shown in supplemental Table 4 according to age at diagnosis. (B) Application of primary therapy among 170 patients (median age, 59 years; interquartile age range, 44-70 years; 79% males; and 87% stage III-IV) diagnosed between 2014 and 2018 according to the exact therapeutic regimen, stratified by age at diagnosis. The absolute number of patients within a specific age and treatment group in panel B is shown in supplemental Table 5. Of note, 17 (10%) of 170 patients diagnosed between 2014 and 2018 received a variety of regimens. These exact regimens are presented in supplemental Table 6. DA, dose-adjusted; EPOCH-R, rituximab with etoposide, vincristine, and doxorubicin; R-IVAC, rituximab, ifosfamide, etoposide, and high-dose cytarabine; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CODOX-M, rituximab, cyclophosphamide, doxorubicin, vincristine, and methotrexate; SCT, stem cell transplantation.
Figure 1B and supplemental Tables 5 and 6 show detailed data on primary therapy according to age at diagnosis among 170 patients diagnosed during 2014 and 2018, of whom 55% were managed in an academic setting. Most patients aged 18 to 75 years received multiagent chemotherapy (100% of patients 18-39 years, 95% of patients 40-59 years, and 86% of patients 60-75 years). Most patients aged >75 years received no treatment (58%), followed by a variety of less intensive regimens.
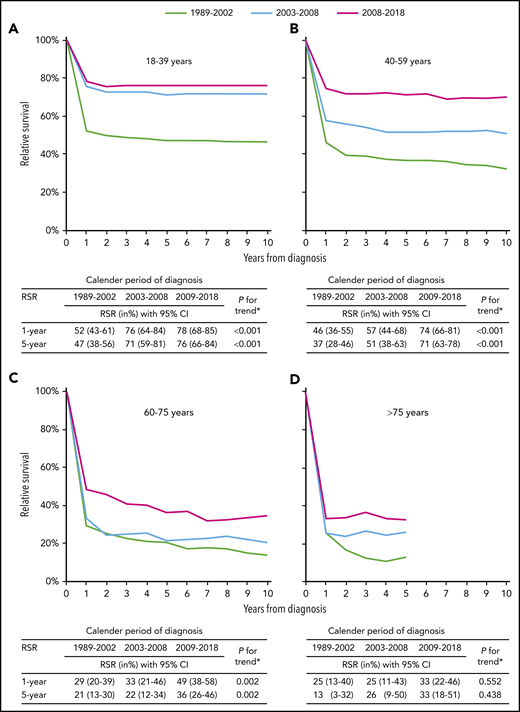
RS according to age at diagnosis and epoch of diagnosis is shown in Figure 2. Between 1989 to 2002 and 2009 to 2018, 1- and 5-year RS increased across all age groups. However, statistically significant improvements were confined to patients aged 18 to 75 years. Encouraging is the plateau in RS 2 years after diagnosis among patients diagnosed from 2009 to 2018. This finding, however, appears less pronounced for patients aged 60 to 75 years. The plateau indicates no further excess mortality compared with equivalent groups from the general population, which is illustrated by a landmark analysis measured from 2 years after diagnosis (supplemental Figure 2). Last, 5-year RS was higher among recipients of rituximab with chemotherapy than recipients of chemotherapy alone (69% vs 38% during 2009-2018; P < .001; supplemental Figure 3).
Relative survival of adult patients with Burkitt lymphoma in The Netherlands, 1989 to 2018. Relative survival rates (RSRs) are presented for the following age categories: (A) 18 to 39, (B) 40 to 59, (C) 60 to 75, and (D) >75 years. The table presents the projected 1- and 5-year RSRs with 95% confidence intervals according to age at diagnosis and calendar period of diagnosis. The asterisk indicates the P value for likelihood ratio test assessing linear trends from the calendar period 1989 to 2002 to the calendar period 2009 to 2018. RSRs for patients aged >75 years were truncated at 5 years, because comparatively few were alive 5 years after diagnosis.
Relative survival of adult patients with Burkitt lymphoma in The Netherlands, 1989 to 2018. Relative survival rates (RSRs) are presented for the following age categories: (A) 18 to 39, (B) 40 to 59, (C) 60 to 75, and (D) >75 years. The table presents the projected 1- and 5-year RSRs with 95% confidence intervals according to age at diagnosis and calendar period of diagnosis. The asterisk indicates the P value for likelihood ratio test assessing linear trends from the calendar period 1989 to 2002 to the calendar period 2009 to 2018. RSRs for patients aged >75 years were truncated at 5 years, because comparatively few were alive 5 years after diagnosis.
The age-stratified multivariable analysis, which was simultaneously adjusted for the epoch of diagnosis, sex, and stage, demonstrated an adverse prognostic effect of advanced disease stage, but not sex, across all age groups (supplemental Table 7). This analysis also demonstrated that statistically significant improvements in survival in the rituximab era (ie, between 2003-2008 and 2009-2018) were restricted to patients aged 40 to 75 years.
In this nationwide population-based study spanning 30 years, RS of BL patients aged 18 to 75 years improved significantly over time. This improvement was, however, less pronounced for patients aged 18 to 39 years in the rituximab era. Nevertheless, 5-year RS in this age group is high (76% during 2009-2018). Interestingly, 5-year RS among patients aged 40 to 59 years reached the level of those aged 18 to 39 years from 2009 to 2018 (71% vs 76%). In the study by Evens et al, patients aged 40 to 59 years had inferior survival compared with those aged 18 to 39 years from 2009 to 2018. We did, however, similar to Evens et al, confirm the benefit of rituximab added to chemotherapy, albeit with a more significant effect than in the RCT.2
The outcome of patients aged >75 years remained comparatively poor over the past 3 decades, even in the rituximab era. However, a particular older patient subset might benefit from treatment because excess mortality was not heightened 1 year after diagnosis from 2009 to 2018. This finding could not be gleaned by the study of Evens et al because this age group was clustered into the age category ≥60 years.
The strengths of our study include the use of a nationwide cancer registry that includes more patients treated in a nonacademic setting and more older patients compared with the registry of Evens et al. Moreover, we report RS instead of OS data. Limitations mainly pertain to the lack of detailed data on prognostic factors. Therefore, we cannot validate the prognostic model proposed by Evens et al. Despite these limitations, we demonstrate that approximately 75% of real-world BL patients aged 18 to 59 years can look forward to a normal life expectancy 2 years after diagnosis. Likewise, a plateau in RS is also observed for subsets of patients >75 years. These data should encourage the design of future intervention studies with alternative, less-toxic regimens for older-aged BL patients.
For original data, email the corresponding author.
The online version of this article contains a data supplement.
Authorship
Contribution: A.G.D. and M.E.D.C. designed the research; D.E.I., R.L.S., A.G.D., and M.E.D.C. analyzed data and wrote the paper; and O.V., S.Z., and P.J.L. reviewed the data and the paper.
Conflict-of-interest disclosure: S.Z. is on the advisory committee for Janssen, Takeda, Celgene, Sanofi, and Oncopeptides and receives research support from Takeda and Janssen. P.J.L. is on the advisory committee for Servier, Takeda, Genmab, Celgene, Incyte, Genentech, and Regeneron and receives research support from Servier and Takeda. M.E.D.C. receives research support from Gilead, GenMab, and BMS. The remaining authors declare no competing financial interests.
Correspondence: M. E. D. Chamuleau, Department of Hematology, Cancer Center Amsterdam, Amsterdam University Medical Centers, VU Medical Center, De Boelelaan 1117, 1081 HV, Amsterdam, The Netherlands; e-mail: m.chamuleau@amsterdamumc.nl.
REFERENCES
Author notes
D.E.I. and R.L.S. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal