In this issue of Blood, Nair et al1 demonstrate that AAV-directed gene therapy using a new bioengineered FIX transgene provides higher FIX activity and superior hemostatic efficacy than other FIX variants and may allow for lower and potentially safer vector doses in future human clinical trials.
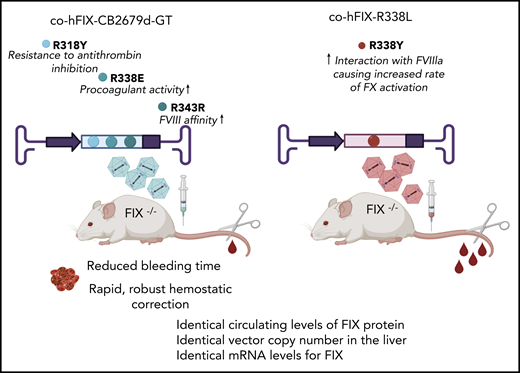
Adult hemophilia B mice were treated with identical AAV vectors, encoding either the FIX CB 2679d-GT or R338L-Padua variants, at the same dose. Evaluation of treated animals demonstrated nearly identical circulating levels of FIX protein, liver vector copy number, and FIX mRNA levels. However, the new CB 2679d/ISU304 transgene, which is designed to have improved FIX functions such as catalytic activity, affinity for activated FVIII, and resistance to antithrombin inhibition, outperformed the R338L-Padua by providing rapid and robust hemostatic correction. Thus, the FIX variant CB 2679d-GT could improve tolerability of the vector in future gene therapy trials.
Adult hemophilia B mice were treated with identical AAV vectors, encoding either the FIX CB 2679d-GT or R338L-Padua variants, at the same dose. Evaluation of treated animals demonstrated nearly identical circulating levels of FIX protein, liver vector copy number, and FIX mRNA levels. However, the new CB 2679d/ISU304 transgene, which is designed to have improved FIX functions such as catalytic activity, affinity for activated FVIII, and resistance to antithrombin inhibition, outperformed the R338L-Padua by providing rapid and robust hemostatic correction. Thus, the FIX variant CB 2679d-GT could improve tolerability of the vector in future gene therapy trials.
Hemophilia B, a bleeding disorder caused by a deficiency in blood coagulation factor IX (FIX), occurs as a result of F9 gene mutations. Although prophylactic therapy with FIX protein is effective in preventing bleeding episodes, the requirement for frequent intravenous infusions, development of inhibitors to FIX, and fluctuations in clotting factor levels all underscore the importance of developing novel approaches for the treatment of these patients. Gene-replacement therapy with FIX transgenes promises cost-effective, long-term solutions that address many limitations of traditional protein-based therapies. The first in-human gene therapy trial for hemophilia B, using adeno-associated viral (AAV) vectors, targeted the muscle, expressed wild-type FIX (FIX-WT), and gave the first glimpse of the tremendous potential of this approach. Alas, although safety and absence of immune responses to AAV and/or the transgene were demonstrated, the lack of systemic FIX expression was disappointing.2 Because FIX is produced in the liver, the next phase 1/2 clinical trial used hepatic-directed delivery of an AAV2-FIX under control of a liver-specific promoter. Although the therapy was transiently successful at higher doses, the therapeutic benefit was lost and was associated with a concomitant increase in markers of liver damage.3 The identification of alternate serotypes of AAV that exhibit natural tropism for the liver paved the way for subsequent clinical gene therapy trials in which the vector was infused into the peripheral circulation.4 Collectively, the results of these trials showed that some subjects in the high-dose cohorts maintain low, but therapeutic, FIX levels for multiple years after AAV infusion. However, they also stressed the vector dose-dependent nature of cytotoxic T-cell responses against AAV capsid proteins displayed on transduced hepatocytes.5 Immune-mediated destruction of these hepatocytes clinically manifested as an asymptomatic increase in plasma liver enzymes (alanine transaminase [ALT]) and led to a loss of circulating FIX protein, unless prednisone was initiated within 48 hours of noting the increase in ALT. A further limitation to IV administration of recombinant AAV is the barrier posed by preexisting immunity against AAV, resulting from natural exposure to WT viruses, which can, depending upon the serotype, lead to rapid clearance of the infused AAV vector. These clinical trials thus underscored the formidable immunologic obstacles that limit the efficacy of systemic AAV gene transfer and led investigators in the field to begin seeking novel means of decreasing the AAV vector dose, to limit the capsid-directed cellular immune responses, while preserving therapeutic efficacy by trying to boost FIX expression and/or activity levels. A major breakthrough came with the identification of the hyperactive FIX Padua.6 This single point mutation (R338L) produces a FIX protein with an eightfold increase in specific activity compared with FIX-WT. The enhanced efficacy afforded by using a FIX-R338L-Padua transgene, compared with FIX-WT, was shown in a clinical trial when subjects demonstrated sustained FIX activity levels of ∼25% of normal without any serious adverse effects.7 As in prior trials, 2 of 10 subjects developed an anti-capsid immune response, but rapid steroid initiation enabled therapeutic FIX activity levels to be maintained. This trial7 provided critical proof-of-concept for the benefit of using a transgene encoding a FIX protein with enhanced activity, as the patients in this trial exhibited approximately fivefold higher FIX activity levels at a fourfold lower vector dose than patients in the earlier trials who received the same vector via the same route. Importantly, the ability to use this lower vector dose also decreased the incidence of subjects needing steroids from 66% to 20%. From the standpoint of patient safety and clinical efficacy, however, one of the much-needed solutions is to find ways to further reduce the vector dose. The study by Nair et al brings exactly that, a new “FIX” for hemophilia B gene therapy.
Using a new transgene, CB 2679d/ISU304 FIX, created by rational protein engineering to improve 3 key functions of FIX, its catalytic activity, its affinity for activated FVIII, and its resistance to antithrombin inhibition, Nair et al sought to enable further reduction in AAV vector dose while enhancing therapeutic efficacy and improving safety. They compared, head-to-head, identical AAV vectors encoding either the Padua or the new CB 2679d/ISU304 FIX transgene at identical doses in hemophilia B mice and subsequently analyzed FIX protein levels by enzyme-linked immunosorbent assay, FIX activity levels by activated partial thromboplastin time, phenotypic correction by tail-clip assay, messenger RNA (mRNA) levels by quantitative real-time polymerase chain reaction (qPCR), and vector distribution and liver copy number by qPCR. They demonstrated that administering the same dose of the 2 vectors results in nearly identical circulating levels of FIX protein, vector copy number in the liver, and mRNA levels for FIX, but that the levels of FIX activity and the hemostatic correction is markedly different between the 2 vectors, with the new CB 2679d/ISU304 transgene yielding highly significant improvements in all parameters at the same vector dose and therapeutic efficacy at a lower vector dose than with the Padua transgene (see figure). As such, the evidence is compelling that this new variant could improve tolerability of the vector and bring us closer to the full realization of safe curative therapies for hemophilia B.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal