In this issue of Blood, Yuan et al demonstrated that cannabinoids modulate the adoptive and innate immune responses involved in the regulation of acute and chronic graft-versus-host disease (GVHD) after allogeneic stem cell transplantation (SCT).1
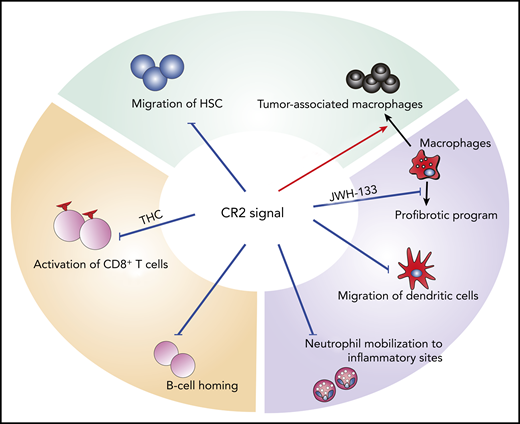
Effects of CR2 signaling on immune and hematopoietic cells. CR2 signaling inhibits migration of HSC, neutrophils, and dendritic cells, as well as CD8+ T-cell activation, and B-cell homing. It inhibits the profibrotic program of macrophages, but augments immune suppressive function of macrophages.
Effects of CR2 signaling on immune and hematopoietic cells. CR2 signaling inhibits migration of HSC, neutrophils, and dendritic cells, as well as CD8+ T-cell activation, and B-cell homing. It inhibits the profibrotic program of macrophages, but augments immune suppressive function of macrophages.
Cannabinoids are a group of chemical substances found in cannabis derived from the plant Cannabis sativa. In addition to the well-known effects to the nervous system through cannabinoid receptor type 1 (CR1), these compounds play significant roles in the regulation of the immune system through cannabinoid receptor type 2 (CR2).2 Accumulating data suggest that they may be useful in the treatment of various disorders involving dysregulated immunity, such as autoimmune diseases, infectious diseases, and cancers.2
Given their immune modulatory properties, cannabinoids have been considered for prophylaxis of allograft rejection and GVHD in transplantation medicine. Tetrahydrocannabinol (THC) and cannabidiol (CBD), which act as selective CR2 agonists, are the most extensively studied cannabinoids. Initial studies evaluating the effects of these cannabinoids in mouse models of GVHD showed a reduction of donor T-cell alloresponses and an amelioration of GVHD.3,4 Based on these observations, a phase 2 trial of CBD was tested in Israel and showed safety and promising efficacy when CBD was combined with standard GVHD prophylaxis.5 However, a better understanding of cannabinoid effects on the immune system is warranted before exploring these compounds further in the clinic. Specifically, there are numerous cannabinoids with complex interactions with multiple receptors and channels. A deeper understanding of these compounds is needed to rationally evaluate their potential.
Yuan et al extensively investigated the role of CR2 in GVHD using mouse models of allogeneic SCT. Acute GVHD was exacerbated in the absence of CR2 on donor T cells or by the administration of a selective CR2 antagonist, suggesting that CR2 signaling in donor T cells, particularly CD8+ T cells, regulated acute GVHD. Interestingly, CR2 expression was downregulated on T cells, but not on macrophages in GVHD. Administration of THC mitigated acute GVHD, where T cells are the major player, but not chronic GVHD. In contrast, another CR2 agonist, JWH-133, ameliorated sclerodermatous chronic GVHD, where macrophages play an important role in fibrosis, but did not impact acute GVHD. These results highlight the complexity of therapeutic targeting of CR2, depending on both agonist characteristics and the immune effector cell types involved in the disease pathogenesis.
However, many questions still remain before the clinical application of this class of compounds. CR2 expression differed significantly among immune cell types and among their resident tissues. Likewise, the effects of inflammation on CR2 expression differ between cell types and tissue types in animal models. These effects are largely unknown in humans. CR2 is highly expressed on B cells and is critical for their homing and function.2 B cells also contribute to human chronic GVHD pathophysiology but play a minor role in the model used in this study. The role of B-cell CR2 in chronic GVHD needs to be elucidated using other models.
CR2 plays a key regulatory role in the function and mobilization of neutrophils6 that may infiltrate the gut and play a role in GVHD pathogenesis.7 Thus, further studies are warranted on the interplay of the intestinal microenvironment, microbiota, and immune responses. CR2 signaling also plays an important role in the migration of hematopoietic stem and progenitor cells, as well as lymphoid reconstitution after SCT.4,8 CR2 augments immunosuppressive function of tumor-associated macrophages9 and may have impacts on graft-versus-tumor effects (see figure).
Many cannabinoids also act as agonists of other G protein–coupled receptors and peroxisome proliferator activated receptor, which may play a role in GVHD. THC also increases adenosine, which could act as a damage-associated molecular pattern, a stimulator of host and donor-derived antigen-presenting cells, which in turn activate and amplify the responses of alloreactive donor T cells, thereby enhancing GVHD. This study is just the beginning of understanding the mechanistic impact of the cannabinoids on GVHD.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal