In this issue of Blood, Yu et al1 report a novel CD44-CXCL12–mediated signaling pathway that leads to resistance to venetoclax, an increase in stem cell features, and a decrease in apoptosis in acute myeloid leukemia (AML).
The painting The Squatter, by the American painter George Caleb Bingham (1811-1879), beautifully depicts a group of squatters, sitting outside their log cabin by a campfire overlooking the North American prairie. While the squatters claimed new territory and took up residence in remote parts of the land, they eventually relinquished them, leaving their abodes to new settlers. The squatters were pioneers, instrumental in pushing the frontier to the West and were renowned for their independence.
Leukemic stem cells (LSCs) may also be considered squatters in their niche in the bone marrow microenvironment (BMM), which is the usual home of normal hematopoietic stem and progenitor cells. There, the LSCs roam, while interacting with neighboring cells, matrices, and soluble factors. Leukemia cells modify this niche in a fashion most conducive to their own survival, allowing their progeny to expand their borders beyond the bone marrow and into the peripheral blood.2 Lodging in this niche also provides a shelter from external threats such as radiation, chemotherapy, or other destructive treatments.3,4
Another external threat to leukemia cells is venetoclax, which belongs to the novel class of BH3-mimetics, which inhibits the anti-apoptotic machinery of (cancer) cells.5 Venetoclax specifically and selectively inhibits the B-cell lymphoma-2 (Bcl-2) protein, leading to programmed death of chronic lymphocytic leukemia and AML cells with good clinical results. In elderly patients with AML, treatment with venetoclax is frequently combined with azacytidine.5 Favorable outcomes have also been reported in preclinical or clinical trials in non-Hodgkin lymphoma, B-cell acute lymphoblastic leukemia, and multiple myeloma.6
As with most successful novel therapies7 much effort is now being placed on understanding mechanisms leading to resistance to venetoclax. Indeed, gene and protein expression studies have revealed that overexpression of the antiapoptotic proteins BCL-XL and MCL-1, either alone or in combination, where neither is targeted by venetoclax, results in resistance. Concomitantly or exclusively, a decrease in proapoptotic proteins or a mutation in the BH3-binding domain (F104L), which may interfere with venetoclax binding, has also been observed,8 but the search for further mechanisms of resistance to such a promising and widely applicable form of treatment is far from over.
In fact, ∼20% of patients with AML treated with a combination of venetoclax and hypomethylating agents are refractory, and other patients, even those achieving a complete remission, may relapse because of the presence of minimal residual disease (MRD). MRD is attributed to a population of leukemia cells that are resistant to specific therapies because of the acquisition of survival advantages with the development of stem cell characteristics and which are usually maintained in the protective BMM. Several pathways, including the integrins, but also the glycoprotein adhesion molecule CD44 on AML cells,9 which binds to the extracellular matrix protein hyaluronan and other ligands, as well as the CXCR4-CXCL12 (stromal-derived factor-1α [SDF1α]) axis, have been shown to mediate the BMM resistance of AML cells to conventional therapies.2
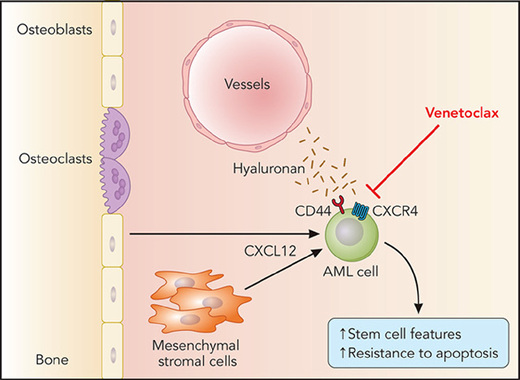
In this study, Yu et al unravel a novel pathway involving the cooperative function of CD44 and CXCR4 (the receptor for BMM-derived CXCL12) that resulted in promotion of stemness features in AML cells and resistance to venetoclax (see figure). Using elegant biomolecular fluorescence technology, they demonstrate that CD44 and CXCR4 form a complex on the cell membrane after stimulation with CXCL12 and that reduced expression of CD44 abrogates CXCL12-mediated induction of the embryonic stem cell core transcription factors Sox2, Oct4, and Nanog in venetoclax-resistant AML cell lines. Venetoclax treatment selected for these stemlike AML cells, which highly express CD44 and are characterized by increased resistance to apoptosis. With the help of a novel xenograft model of AML in zebrafish, Yu et al show in vivo that deficiency of CD44 may restore sensitivity of AML cells to venetoclax, suggesting that CD44-blocking strategies, which have been attempted in the past in preclinical studies for AML9 and chronic myeloid leukemia,10 may be a beneficial tool to circumvent or ameliorate resistance to venetoclax.
BMM-derived CXCL12 leading to cooperativity between CXCR4 and CD44 on the leukemic stem cell in acute myeloid leukemia (AML) and resistance to venetoclax by an increase in expression of CD44 and the embryonic stem cell core transcription factors Sox2, Oct4, and Nanog.
BMM-derived CXCL12 leading to cooperativity between CXCR4 and CD44 on the leukemic stem cell in acute myeloid leukemia (AML) and resistance to venetoclax by an increase in expression of CD44 and the embryonic stem cell core transcription factors Sox2, Oct4, and Nanog.
In sum, Yu et al elucidate a novel AML cell–extrinsic mechanism of resistance to venetoclax, leading to the promotion of AML cells with stem cell properties related to CD44 expression. Although CD44 deficiency can affect MCL-1 expression and inhibition of MCL-1 sensitizes AML cells to venetoclax, synergistic effects of this three-pronged approach, meaning inhibition of CD44, MCL-1, and venetoclax, must be tested in future studies. It is a daunting thought that the cancer stem cell marker CD44 may also be involved in resistance to treatments for solid cancers via its binding to hyaluronan, osteopontin, or E-selectin. Similarly, how hyaluronan, which can also bind to CXCL12 directly or how other extracellular matrix proteins may participate in the induction of stemness features via CD44 and/or other receptors on AML cells, remains to be clarified. Insight into and, eventually, perturbation of BMM-usurping mechanisms would surely hand the squatters their final eviction notice.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal