Key Points
Danicopan, a first-in-class oral factor D inhibitor showed clinical benefit when given in addition to eculizumab in patients with PNH.
Addition of danicopan reduced extravascular hemolysis and improved anemia in patients with PNH with suboptimal eculizumab response.
Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is characterized by uncontrolled terminal complement activation and subsequent intravascular hemolysis (IVH). C5 inhibitors prevent membrane attack complex formation, but patients may experience extravascular hemolysis (EVH) and continue to require blood transfusions. Danicopan, an oral proximal complement inhibitor of alternative pathway factor D (FD), is designed to control IVH and EVH. In a phase 2 dose-finding trial, eculizumab-treated transfusion-dependent patients with PNH (n = 12) received danicopan, 100 to 200 mg thrice daily, in addition to their eculizumab regimen for 24 weeks. End points included hemoglobin (Hgb) change vs baseline at week 24 (primary), reduction in blood transfusions, and patient-reported outcomes. Safety, tolerability, and pharmacokinetics/pharmacodynamics were measured. Twelve patients received ≥1 danicopan dose; 1 patients discontinued from a serious adverse event deemed unlikely related to danicopan. Eleven patients completed the 24-week treatment period. Addition of danicopan resulted in a mean Hgb increase of 2.4 g/dL at week 24. In the 24 weeks prior to danicopan, 10 patients received 31 transfusions (50 units) compared with 1 transfusion (2 units) in 1 patient during the 24-week treatment period. Mean Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue score increased by 11 points from baseline to week 24. The most common adverse events were headache, cough, and nasopharyngitis. Addition of danicopan, a first-in-class FD inhibitor, led to a meaningful improvement in Hgb and reduced transfusion requirements in patients with PNH who were transfusion-dependent on eculizumab. These benefits were associated with improvement of FACIT-Fatigue. This trial was registered at www.clinicaltrials.gov as #NCT03472885.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare disease; estimates suggest a worldwide prevalence of 1.59 per 100 000 people, with ∼1.3 new cases per million people per year.1 It has been suggested that PNH, like aplastic anemia, may be more frequent in Southeast and East Asia.2
PNH is caused by a somatic mutation in the phosphatidylinositol N-acetylglucosaminyltransferase subunit A gene in ≥1 hematopoietic stem cell that results in loss of glycosylphosphatidylinositol (GPI)-anchored proteins, including complement regulatory proteins CD55 and CD59, from the surface of affected cells. Clonal expansion results in an increased population of cells lacking CD55 and CD59 that are vulnerable to intravascular hemolysis (IVH) mediated by the membrane attack complex (MAC), a product derived from terminal complement activation.3,4
In addition to having anemia that may require frequent red blood cell (RBC) transfusions, patients with PNH are at high risk for thrombotic events usually associated with intravascular hemolysis (IVH), which can be a major cause of morbidity and mortality.5 Patients may experience smooth muscle dysfunction (eg, dysphagia, erectile dysfunction, abdominal pain), presumably related to the release of hemoglobin (Hgb) from RBCs and the consequent derangement of vasculature nitric oxide levels.
Approval of the C5 inhibitor monoclonal antibody eculizumab (Soliris), which blocks terminal complement activation and subsequent IVH, revolutionized the treatment of PNH. Patients with PNH who received eculizumab achieved control of IVH-related symptoms, improved quality of life, markedly reduced thromboembolic events typical of PNH, and substantially improved survival.6-10 Although eculizumab is a life-changing therapy, the majority of patients experience residual anemia, and a small subset of these remain transfusion dependent.11-16 In some cases, residual anemia may result from transient low eculizumab concentrations immediately prior to the next dosing cycle, which can be overcome by increasing the dose.17-19 In most cases, anemia may be attributed to a lack of inhibitory effect of eculizumab on proximal complement causing extravascular hemolysis (EVH) in patients with PNH treated with C5 inhibition. Because of the continuous activation of complement alternative pathway (AP) by “tick-over” (ie, the slow spontaneous hydrolysis of the C3 thioester), C3 fragments continuously accumulate on GPI-deficient erythrocytes while circulating in the blood, even if they are spared from IVH because of eculizumab blockade of terminal complement activation at C5.11-14,20,21 These C3 fragment opsonized cells may be destroyed via EVH by hepatosplenic phagocytes.11,20 This process is clinically relevant in the subgroup of treated patients presenting with residual anemia and transfusion dependence, as initially reported in the literature.11 Therefore, a need exists in this patient population for treatment that may improve the symptomatic anemia that some patients continue to experience.
Danicopan, a first-in-class small molecule orally administered complement factor D (FD) inhibitor, is being developed for the treatment of PNH and other complement-mediated diseases.22,23 FD, a serine protease, catalyzes the cleavage of complement factor B into Ba and Bb, allowing the formation of the AP C3 convertase (C3bBb) (Figure 1). Because FD catalyzes a rate-limiting step of AP activation and amplification and is among the lowest plasma concentrations of all complement plasma proteins, it was chosen as a therapeutic target. By inhibiting FD serine protease activity, danicopan specifically targets the control point of the complement cascade amplification loop, blocking C3 convertase formation and, therefore, significantly reducing production of C3 cleavage products (also known as C3 fragments) and downstream MAC formation. Although danicopan does not inhibit components specific to the classical pathway (CP) or lectin pathway (LP) or components of the terminal complement pathway, it will inhibit the AP-mediated amplification of complement activity initiated via the CP and LP.24 A phase 2 trial demonstrated the efficacy and safety of danicopan monotherapy in untreated patients with PNH; however, some patients experienced IVH,25 suggesting that clinical effect could be improved by implementing treatment with a C5 inhibitor to block IVH (mediated by activation of complement terminal pathway) while addressing EVH (likely via C3 fragment opsonization) through danicopan add-on therapy.
Inhibition of FD proteolytic activity by danicopan. FD participates in C3 convertase generation by cleavage of factor B at 2 steps in the AP cascade: generation of the initial C3 convertase [C3(H2O)Bb] following spontaneous AP activation (tick-over) in the fluid phase and the production of surface-bound C3 convertase (C3bBb), which mediates dramatic amplification of the initial activation (amplification loop) and activation of the terminal pathway (cleavage of C5 into C5a and C5b by C5 convertase [ie, C3bBb3b]), leading to opsonization of target surfaces by C3b, release of the anaphylatoxins C3a and C5a, and formation of MAC. An erythrocyte is shown to depict the membrane-bound events. Regulatory proteins not shown here can promote (properdin and FH-related proteins) or attenuate (factor H, factor I, multiple membrane-bound proteins, including CD55 and CD59) AP activity. Eculizumab acts directly at the terminal pathway by preventing its activation via binding to C5; danicopan acts upstream by preventing AP activation via inhibition of proteolytic activity of FD. Although the C5 inhibitor is able to block MAC formation regardless of the initiation pathway of complement activation, it has no effect on C3 fragment deposition. FD inhibitor is able to block C3 fragment deposition and MAC formation when the complement activation is initiated via AP, such as in the case of PNH; it should be able to exert the same, although not complete, inhibitory effect when complement activation is initiated via CP or LP through blocking the amplification loop.24
Inhibition of FD proteolytic activity by danicopan. FD participates in C3 convertase generation by cleavage of factor B at 2 steps in the AP cascade: generation of the initial C3 convertase [C3(H2O)Bb] following spontaneous AP activation (tick-over) in the fluid phase and the production of surface-bound C3 convertase (C3bBb), which mediates dramatic amplification of the initial activation (amplification loop) and activation of the terminal pathway (cleavage of C5 into C5a and C5b by C5 convertase [ie, C3bBb3b]), leading to opsonization of target surfaces by C3b, release of the anaphylatoxins C3a and C5a, and formation of MAC. An erythrocyte is shown to depict the membrane-bound events. Regulatory proteins not shown here can promote (properdin and FH-related proteins) or attenuate (factor H, factor I, multiple membrane-bound proteins, including CD55 and CD59) AP activity. Eculizumab acts directly at the terminal pathway by preventing its activation via binding to C5; danicopan acts upstream by preventing AP activation via inhibition of proteolytic activity of FD. Although the C5 inhibitor is able to block MAC formation regardless of the initiation pathway of complement activation, it has no effect on C3 fragment deposition. FD inhibitor is able to block C3 fragment deposition and MAC formation when the complement activation is initiated via AP, such as in the case of PNH; it should be able to exert the same, although not complete, inhibitory effect when complement activation is initiated via CP or LP through blocking the amplification loop.24
This report presents data from the 24-week primary treatment period of the phase 2 study assessing the safety and effectiveness of danicopan in addition to eculizumab. The patient population studied had an inadequate response to eculizumab monotherapy, as demonstrated by continued transfusion dependency.
Methods
Study design
This is a multiple-center open-label multiple-dose phase 2 study in patients with PNH with an inadequate response to eculizumab treatment defined as ≥1 RBC transfusion within 12 weeks prior to screening. During the primary treatment period, patients received 24 weeks of danicopan treatment thrice daily orally plus eculizumab administered at their usual dose and schedule. Patients who completed the 24-week primary treatment period with clinical benefit could enter a long-term extension phase in which they would continue to receive danicopan and eculizumab. This trial was approved by regulatory agencies/local ethics committees and conducted according to the International Conference on Harmonization and Good Clinical Practice Standards. Achillion, Inc., now a wholly-owned subsidiary of Alexion Pharmaceuticals, Inc., designed and sponsored the study, with input from investigators. All participants provided written informed consent.
Patients
The primary treatment period took place from June of 2018 to September of 2019. Patients aged from 18 to 65 years with PNH and RBC transfusion-dependent anemia, defined as having received ≥1 RBC transfusion within 12 weeks prior to screening, were eligible for the study. Patients were required to be receiving a stable dose of eculizumab, defined as an approved or higher dose for ≥24 weeks prior to entry without a change in dose or schedule for ≥8 weeks prior to screening. Additional criteria included Hgb < 10 g/dL (and adequate reticulocytosis), platelets ≥ 40 000 per microliter, and a willingness to receive Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae vaccinations.
Treatment
Patients received oral danicopan (ACH-4471; ACH-0144471; ALXN2040) at a starting dose of 100 mg or 150 mg, thrice daily, and were instructed to take doses at approximately the same time each day and as close as possible to 8 hours apart; dosing interval was informed by the ∼9-hour half-life of danicopan.22 Dose escalations, permitted at 4-week intervals to a maximum of 200 mg, thrice daily, in 50-mg increments, were based on safety and Hgb levels through week 12. If the patient had not reached the 200-mg thrice-daily maximal dose by week 12, escalation was permitted if clinically indicated. These starting doses were based on previous assessments of danicopan in healthy human volunteers and in vitro assays using erythrocytes from patients with PNH.22,23 Patients continued their preexisting IV regimen of eculizumab. Switching from eculizumab to ravulizumab was not permitted during the treatment period; however, patients who entered the long-term extension phase were subsequently permitted to transition to ravulizumab (ULTOMIRIS).
End points
The primary end point was efficacy of danicopan in addition to eculizumab based on Hgb increase at week 24 relative to baseline. Secondary objectives included reductions in (1) RBC transfusions, including units transfused, during the 24 weeks of danicopan compared with the 24 weeks prior to danicopan, (2) the percentage of patients who were transfusion independent during the 24 weeks of treatment, (3) lactate dehydrogenase (LDH) levels at week 24 compared with baseline. Additional end points included effect of danicopan on complement biomarkers and assessment of fatigue with the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue instrument; total scores range from 0 to 52, with the higher scores indicating an improvement.26 Safety, tolerability, and danicopan plasma concentration were also measured.
Pharmacokinetics and pharmacodynamics methods
Additional information regarding assay methods is presented in supplemental Assay methods (available on the Blood Web site). Plasma danicopan concentrations were determined by liquid chromatography. Pharmacodynamics was determined by measuring serum AP activity with an AP hemolysis assay. Serum CP activity, as well as plasma Bb and serum FD and C3 concentrations, were monitored. With the exception of the AP hemolysis assay, which was conducted internally for exploratory purposes using previously described methods,24 complement tests were conducted by a central laboratory using commercial kits. At each AP hemolysis run, a single normal human serum sample was included in addition to the patient serum samples so that hemolysis values of individual samples were able to be standardized to the hemolysis value of the normal sample. Last, GPI-deficient cell population sizes of granulocytes and erythrocytes were measured using flow cytometry; C3 fragment deposition on GPI-deficient erythrocytes was measured using flow cytometry with an FITC-conjugated anti-human C3d antibody (Assay Pro; cat. #11294-05041).
Statistical methods
This exploratory phase 2 study is the first evaluation of danicopan add-on therapy in patients receiving eculizumab background therapy; as such, the sample size was small, and biochemical, FACIT-Fatigue, and transfusion data were summarized using descriptive statistics. Continuous variables were summarized as mean and standard deviation. The change in numeric efficacy end points (hemoglobin, LDH, reticulocyte count, direct and total bilirubin, and FACIT-Fatigue scores) from baseline to week 24 were analyzed using a 1-sample Student t test. Descriptive statistics, including the number of patients, mean, and coefficient of variation were used to summarize the calculated pharmacokinetics (PK) parameters. Categorical variables (eg, transfusion independence) were summarized with counts and percentages. Missing values were not imputed.
Results
Patient characteristics
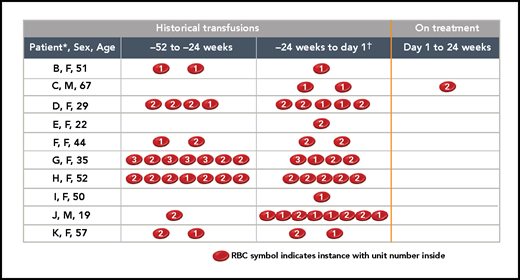
Twelve patients enrolled in the study and received ≥1 dose of danicopan. One patient discontinued after 2 doses of danicopan because of a serious adverse event that was considered unlikely to be related to the study drug (see Study disposition). This patient’s data have been excluded from efficacy analysis. Eleven patients completed the 24-week primary treatment period (Table 1). Median age was 48 years (range, 19-72); protocol exceptions were granted for 2 patients, aged 67 years and 72 years, who otherwise would have been ineligible because of the age criterion. Of the 11 patients included in the efficacy analysis, all were on a stable regimen of IV eculizumab at the start of the study. Eight patients received 900 mg every 14 days, including 1 patient in the United States who had been receiving 1200 mg every 14 days, which was decreased to 900 mg before week 16 because of an insurance requirement resulting from Hgb improvement and who remained on the 900-mg dose throughout the study; 2 patients received 1200 mg every 14 days, and 1 patient received 1500 mg every 14 days. Patients had slightly elevated LDH levels at entry, despite stable eculizumab therapy (mean, 244.5 IU/L; 1.06× upper limit of normal [ULN]). Patients were anemic (mean Hgb, 7.9 mg/dL), and all but 1 had a history of RBC transfusions, with a mean of 2.8 transfusions (mean, 4.5 units) in the 24 weeks prior to the first dose of danicopan. The patient (patient A) without a transfusion history did not accept transfusions because of religious objections. Her baseline Hgb was 5.0 g/dL, and she had an additional diagnosis of genetically confirmed hereditary elliptocytosis.
Key clinical parameters at baseline, weeks 12 and 24
| . | Patients A . | Patient B . | Patient C . | Patient D . | Patient E . | Patient F . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 41 y/o, F . | 51 y/o, F . | 67 y/o, M . | 29 y/o, F . | 22 y/o, F . | 44 y/o, F . | |||||||||||||
| BL . | W12 . | W24 . | BL . | W12 . | W24 . | BL . | W12 . | W24 . | BL . | W12 . | W24 . | BL . | W12 . | W24 . | BL . | W12 . | W24 . | |
| Danicopan, mg oral TID | 100 | 150 | 150 | 100 | 100 | 100 | 150 | 150 | 150 | 150 | 150 | 150 | 100 | 100 | 150 | 100 | 100 | 150 |
| Eculizumab, mg IV q14d | 900 | 900 | 900 | 1200 | 900 | 900 | 900 | 900 | 900 | 900 | 900 | 900 | 1200 | 1200 | 1200 | 900 | 900 | 900 |
| Hgb, g/dL | 5.00 | 7.70 | 8.50 | 9.80 | 11.8 | 13.3 | 7.60 | 9.00 | 9.70 | 10.4 | 11.7 | 11.5 | 8.60 | 7.60 | 9.40 | 7.20 | 9.30 | 10.6 |
| LDH, ×ULN | 1.7 | 1.2 | 1.1 | 1.0 | 0.8 | 0.9 | 0.8 | 0.9 | 0.9 | 1.1 | 1.2 | 0.9 | 1.2 | 1.3 | 1.2 | 0.9 | 0.9 | 0.9 |
| Reticulocytes, 103/μL | 159 | 121 | 112 | 250 | 123 | 162 | 141 | 97.0 | 87.0 | 191 | 56 | 56 | 120 | 179 | 90 | 405 | 243 | 253 |
| Total bilirubin, mg/dL | 2.14 | 1.80 | 2.40 | 1.24 | 0.89 | 0.81 | 1.03 | 0.580 | 0.580 | 2.26 | 0.700 | 0.650 | 2.35 | 2.22 | 1.75 | 3.93 | 1.50 | 2.95 |
| Direct bilirubin, mg/dL | 0.44 | 0.33 | 0.48 | 0.29 | 0.18 | 0.20 | 0.24 | 0.16 | 0.16 | 0.43 | 0.15 | 0.17 | 0.72 | 0.40 | 0.60 | 0.82 | 0.49 | 0.78 |
| GPI-deficient RBC population, % | 57 | 83 | —* | 80 | 89 | —* | 22 | 59 | 51 | 52 | 99 | 100 | 21 | 37 | 53 | 95 | 99 | 98 |
| GPI-deficient granulocyte cell population, % | 84 | 87 | —* | 99 | 85 | —* | 81 | 89 | 98 | 99 | 97 | 99 | 82 | 88 | 82 | 98 | 99 | 99 |
| C3d+ GPI-deficient RBCs, % | 6.30 | —† | 2.20 | 8.20 | —† | 1.00 | 17.5 | 18.6 | 16.7 | 15.9 | 0.200 | 5.20 | 21.3 | 11.8 | 4.90 | 71.0 | 48.0 | 53.4 |
| FACIT-Fatigue‡ | 33 | 48 | 52 | 45 | 49 | 48 | 17 | 36 | 26 | 18 | 41 | 41 | 42 | 25 | 38 | 9 | 48 | 52 |
| . | Patients A . | Patient B . | Patient C . | Patient D . | Patient E . | Patient F . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 41 y/o, F . | 51 y/o, F . | 67 y/o, M . | 29 y/o, F . | 22 y/o, F . | 44 y/o, F . | |||||||||||||
| BL . | W12 . | W24 . | BL . | W12 . | W24 . | BL . | W12 . | W24 . | BL . | W12 . | W24 . | BL . | W12 . | W24 . | BL . | W12 . | W24 . | |
| Danicopan, mg oral TID | 100 | 150 | 150 | 100 | 100 | 100 | 150 | 150 | 150 | 150 | 150 | 150 | 100 | 100 | 150 | 100 | 100 | 150 |
| Eculizumab, mg IV q14d | 900 | 900 | 900 | 1200 | 900 | 900 | 900 | 900 | 900 | 900 | 900 | 900 | 1200 | 1200 | 1200 | 900 | 900 | 900 |
| Hgb, g/dL | 5.00 | 7.70 | 8.50 | 9.80 | 11.8 | 13.3 | 7.60 | 9.00 | 9.70 | 10.4 | 11.7 | 11.5 | 8.60 | 7.60 | 9.40 | 7.20 | 9.30 | 10.6 |
| LDH, ×ULN | 1.7 | 1.2 | 1.1 | 1.0 | 0.8 | 0.9 | 0.8 | 0.9 | 0.9 | 1.1 | 1.2 | 0.9 | 1.2 | 1.3 | 1.2 | 0.9 | 0.9 | 0.9 |
| Reticulocytes, 103/μL | 159 | 121 | 112 | 250 | 123 | 162 | 141 | 97.0 | 87.0 | 191 | 56 | 56 | 120 | 179 | 90 | 405 | 243 | 253 |
| Total bilirubin, mg/dL | 2.14 | 1.80 | 2.40 | 1.24 | 0.89 | 0.81 | 1.03 | 0.580 | 0.580 | 2.26 | 0.700 | 0.650 | 2.35 | 2.22 | 1.75 | 3.93 | 1.50 | 2.95 |
| Direct bilirubin, mg/dL | 0.44 | 0.33 | 0.48 | 0.29 | 0.18 | 0.20 | 0.24 | 0.16 | 0.16 | 0.43 | 0.15 | 0.17 | 0.72 | 0.40 | 0.60 | 0.82 | 0.49 | 0.78 |
| GPI-deficient RBC population, % | 57 | 83 | —* | 80 | 89 | —* | 22 | 59 | 51 | 52 | 99 | 100 | 21 | 37 | 53 | 95 | 99 | 98 |
| GPI-deficient granulocyte cell population, % | 84 | 87 | —* | 99 | 85 | —* | 81 | 89 | 98 | 99 | 97 | 99 | 82 | 88 | 82 | 98 | 99 | 99 |
| C3d+ GPI-deficient RBCs, % | 6.30 | —† | 2.20 | 8.20 | —† | 1.00 | 17.5 | 18.6 | 16.7 | 15.9 | 0.200 | 5.20 | 21.3 | 11.8 | 4.90 | 71.0 | 48.0 | 53.4 |
| FACIT-Fatigue‡ | 33 | 48 | 52 | 45 | 49 | 48 | 17 | 36 | 26 | 18 | 41 | 41 | 42 | 25 | 38 | 9 | 48 | 52 |
| . | Patient G . | Patient H . | Patient I . | Patient J . | Patient K . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 35 y/o, F . | 53 y/o, F . | 51 y/o, F . | 19 y/o, M . | 57 y/o, F . | ||||||||||
| . | BL . | W12 . | W24 . | BL . | W12 . | W24 . | BL . | W12 . | W24 . | BL . | W12 . | W24 . | BL . | W12 . | W24 . |
| Danicopan, mg oral TID | 100 | 100 | 100 | 100 | 200 | 200 | 100 | 150 | 200 | 100 | 100 | 200 | 100 | 100 | 150 |
| Eculizumab, mg IV q14d | 1500 | 1500 | 1500 | 900 | 900 | 900 | 900 | 900 | 900 | 1200 | 1200 | 1200 | 900 | 900 | 900 |
| Hgb, g/dL | 7.10 | 9.40 | 9.10 | 7.70 | 7.30 | 7.50 | 7.70 | 10.8 | 11.5 | 7.80 | 10.2 | 11.0 | 14.7 | 9.40 | 11.5 |
| LDH, ×ULN | 0.6 | 0.5 | 0.9 | 1.5 | 1.8 | 1.4 | 0.9 | 0.9 | 0.9 | 1.3 | —* | 1.2 | 0.9 | 1.2 | 1.1 |
| Reticulocytes, 103/μL | 262 | 200 | 239 | 238 | 206 | 169 | 200 | 110 | 96 | 258 | 292 | 152 | 188 | 88 | 69 |
| Total bilirubin, mg/dL | 1.20 | 1.90 | 1.25 | 2.18 | 2.04 | 1.57 | 0.56 | 0.34 | 0.32 | 3.24 | —* | 1.14 | 3.76 | 1.85 | 1.41 |
| Direct bilirubin, mg/dL | 0.39 | 0.50 | 0.39 | 0.78 | 0.53 | 0.52 | 0.23 | 0.18 | 0.18 | 0.68 | —* | 0.23 | 0.64 | 0.39 | 0.31 |
| GPI-deficient RBC population, % | 63 | 90 | 96 | 19 | 71 | —‡ | 73 | 95 | —* | 60 | 99 | 99 | 55 | 84 | 91 |
| GPI-deficient granulocyte cell population, % | 100 | 100 | 100 | 94 | 95 | —* | 98 | 98 | —* | 99 | 100 | 100 | 75 | 82 | 88 |
| C3d+ GPI-deficient RBCs, % | 80.0 | 29.4 | 57.0 | 38.8 | 14.9 | 22.8 | 13.6 | 0.30 | 0.10 | 34.3 | 57.9 | 28.7 | 20.2 | 44.2 | 6.30 |
| FACIT-Fatigue‡ | 28 | 44 | 38 | 47 | 49 | 49 | 48 | 50 | 49 | 49 | 51 | 51 | 33 | 46 | 50 |
| . | Patient G . | Patient H . | Patient I . | Patient J . | Patient K . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 35 y/o, F . | 53 y/o, F . | 51 y/o, F . | 19 y/o, M . | 57 y/o, F . | ||||||||||
| . | BL . | W12 . | W24 . | BL . | W12 . | W24 . | BL . | W12 . | W24 . | BL . | W12 . | W24 . | BL . | W12 . | W24 . |
| Danicopan, mg oral TID | 100 | 100 | 100 | 100 | 200 | 200 | 100 | 150 | 200 | 100 | 100 | 200 | 100 | 100 | 150 |
| Eculizumab, mg IV q14d | 1500 | 1500 | 1500 | 900 | 900 | 900 | 900 | 900 | 900 | 1200 | 1200 | 1200 | 900 | 900 | 900 |
| Hgb, g/dL | 7.10 | 9.40 | 9.10 | 7.70 | 7.30 | 7.50 | 7.70 | 10.8 | 11.5 | 7.80 | 10.2 | 11.0 | 14.7 | 9.40 | 11.5 |
| LDH, ×ULN | 0.6 | 0.5 | 0.9 | 1.5 | 1.8 | 1.4 | 0.9 | 0.9 | 0.9 | 1.3 | —* | 1.2 | 0.9 | 1.2 | 1.1 |
| Reticulocytes, 103/μL | 262 | 200 | 239 | 238 | 206 | 169 | 200 | 110 | 96 | 258 | 292 | 152 | 188 | 88 | 69 |
| Total bilirubin, mg/dL | 1.20 | 1.90 | 1.25 | 2.18 | 2.04 | 1.57 | 0.56 | 0.34 | 0.32 | 3.24 | —* | 1.14 | 3.76 | 1.85 | 1.41 |
| Direct bilirubin, mg/dL | 0.39 | 0.50 | 0.39 | 0.78 | 0.53 | 0.52 | 0.23 | 0.18 | 0.18 | 0.68 | —* | 0.23 | 0.64 | 0.39 | 0.31 |
| GPI-deficient RBC population, % | 63 | 90 | 96 | 19 | 71 | —‡ | 73 | 95 | —* | 60 | 99 | 99 | 55 | 84 | 91 |
| GPI-deficient granulocyte cell population, % | 100 | 100 | 100 | 94 | 95 | —* | 98 | 98 | —* | 99 | 100 | 100 | 75 | 82 | 88 |
| C3d+ GPI-deficient RBCs, % | 80.0 | 29.4 | 57.0 | 38.8 | 14.9 | 22.8 | 13.6 | 0.30 | 0.10 | 34.3 | 57.9 | 28.7 | 20.2 | 44.2 | 6.30 |
| FACIT-Fatigue‡ | 28 | 44 | 38 | 47 | 49 | 49 | 48 | 50 | 49 | 49 | 51 | 51 | 33 | 46 | 50 |
F, female; M, male; q14d, every 14 days; TID, thrice daily; ULN, upper limit of normal; y/o, years old.
Stability of the sample was interrupted.
C3 fragment deposition was not tested at week 12 for this subject. No data entry.
Scores based on the FACIT-Fatigue Scale V4. Score ranges from 0 to 52; a score < 30 indicates severe fatigue.
Study disposition
Of the 12 patients enrolled, 10 patients started danicopan at 100 mg every 8 hours, and 2 patients started at 150 mg every 8 hours. Of the former group, 7 were titrated to 150 mg, with 3 patients further titrating to 200 mg by the end of the 24-week primary treatment period; no dose-dependent trends were observed with respect to responses or safety/tolerability (see details of dose by visit in supplemental Table 1). Four patients had missed doses, with no effects reported.
All patients maintained their eculizumab regimen throughout the study with the exception of 1 patient (patient B) who had her eculizumab dose decreased, as mandated by her insurance company (from 1200 mg to 900 mg), prior to week 16. One patient experienced an unrelated serious adverse event of pulmonary edema after 2 doses of danicopan (100 mg), resulting in withdrawal of danicopan and early discontinuation from the study; this patient’s data were excluded from efficacy analyses. Safety analysis included data from all 12 patients. All 11 patients who completed the 24-week primary treatment period entered the long-term extension phase, which is ongoing.
Pharmacokinetics
During intensive PK sampling days during week 7, the mean (% CV) values for Cmax, Tmax, and AUC(0-8hr) for danicopan at steady-state were 432 (37) ng/mL, 2.14 (33) hours, and 1806 (37) ng/h per milliliter, respectively. The mean (% CV) Ctrough value was 105 (57) ng/mL during intensive sampling days. Trough sampling over the rest of the study duration under less controlled conditions yielded a mean (% CV) Ctrough value of 150 (59) ng/mL. Results are consistent with PK values for danicopan observed in studies of healthy volunteers.
Clinical efficacy
Primary end point
There was a significant increase in mean Hgb from baseline (7.9 g/dL) to week 24 (10.3 g/dL; increase from baseline, 2.4 g/dL; P = .0001; Figure 2A). This treatment effect appeared by week 2 in most patients and was maintained for the duration of the study.
Effect of danicopan on laboratory markers. Mean (and standard deviation [SD]) change from baseline (BL) for Hgb (primary end point) (A), reticulocytes (B), total bilirubin (C), direct bilirubin (D), LDH (E), and FACIT-Fatigue score (F). P values represent change from baseline to week 24.
Effect of danicopan on laboratory markers. Mean (and standard deviation [SD]) change from baseline (BL) for Hgb (primary end point) (A), reticulocytes (B), total bilirubin (C), direct bilirubin (D), LDH (E), and FACIT-Fatigue score (F). P values represent change from baseline to week 24.
Secondary end points
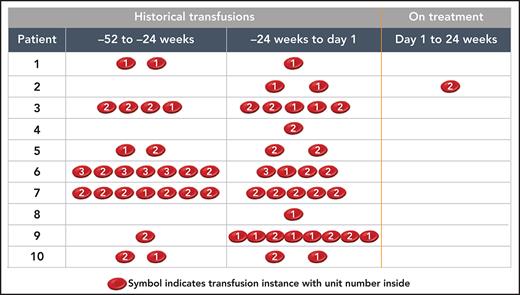
Benefits were observed in multiple PNH laboratory markers (individual outcomes are shown in Table 1; averages for all patients are summarized in Figure 2). Between baseline and week 24, there were decreases in mean absolute reticulocyte counts (from 219 × 103/μL to 135 × 103/μL; P < .0001; Figure 2B), total bilirubin (from 2.17 mg/dL to 1.35 mg/dL; P = .0094; Figure 2C), and direct bilirubin (from 0.51 mg/dL to 0.37 mg/dL; P = .0093; Figure 2D); however, there was no change in the mean LDH level (1.06× ULN at baseline and 1.04× ULN at week 24; Figure 2E). A clinically meaningful reduction in RBC transfusion needs was demonstrated over the 24-week treatment period (Figure 3). Among the 11 patients included in the efficacy analyses, 10 patients received a total of 31 transfusions (50 units) within 24 weeks before the first dose of danicopan. Patient A rejected transfusions because of religious reasons. After commencing danicopan treatment, only 1 patient received a single transfusion (2 units) through week 24, which occurred during hospitalization for pneumonia and was considered unlikely to be related to danicopan by the investigator (Figure 3). This represents a mean reduction of 2.7 transfusions and a mean reduction of 4.4 transfusion units over the 24-week treatment period.
Effect of danicopan on blood transfusions. Per-patient transfusion occurrences and units 52 weeks prior to the start of danicopan and during treatment with danicopan. *Patient A was excluded from the table because of a religious objection to receiving transfusions; baseline Hgb = 5 g/dL.†−24 weeks up to the first dose of study medication.
Effect of danicopan on blood transfusions. Per-patient transfusion occurrences and units 52 weeks prior to the start of danicopan and during treatment with danicopan. *Patient A was excluded from the table because of a religious objection to receiving transfusions; baseline Hgb = 5 g/dL.†−24 weeks up to the first dose of study medication.
Patient-reported outcomes
The mean FACIT-Fatigue score was increased by 11 points (P = .0191), from 34 at baseline (when patients were receiving only eculizumab) to 45 at week 24 (danicopan and eculizumab treatment; Figure 2F). This self-reporting instrument measures the severity of fatigue on a scale of 0 to 52; a score < 30 represents severe fatigue, and higher scores indicate improvement in fatigue.27
Additional end points
Complement biomarkers were monitored during the study. Serum FD and C3 concentrations were normal at baseline with little change during danicopan treatment (data not shown). As expected, inhibition of CP activity was near complete at baseline because of the presence of the C5 inhibitor eculizumab (Figure 4A). This persisted throughout the study. In contrast, residual AP activity was detected with an AP hemolysis assay at baseline; it was reduced further after dosing danicopan (Figure 4B). In parallel, plasma Bb levels were reduced after dosing with danicopan (Figure 4C).
Effect of danicopan on complement biomarkers and GPI-deficient cell population. Serum, plasma, and whole blood samples were collected at day 1 prior to dosing danicopan (baseline [BL]) and at the indicated time points during the study and subjected to measurement of CP activity (A), AP activity with AP hemolysis assay (B), plasma Bb concentration (C), and the population size of GPI-deficient granulocytes, GPI-deficient erythrocytes, and C3d+ GPI-deficient erythrocytes (D), as described in Methods. Arithmetic mean and standard derivation (SD) are shown for all, with the range shown at each time point. LLN, lower limit of normal; NHS, normal human serum.
Effect of danicopan on complement biomarkers and GPI-deficient cell population. Serum, plasma, and whole blood samples were collected at day 1 prior to dosing danicopan (baseline [BL]) and at the indicated time points during the study and subjected to measurement of CP activity (A), AP activity with AP hemolysis assay (B), plasma Bb concentration (C), and the population size of GPI-deficient granulocytes, GPI-deficient erythrocytes, and C3d+ GPI-deficient erythrocytes (D), as described in Methods. Arithmetic mean and standard derivation (SD) are shown for all, with the range shown at each time point. LLN, lower limit of normal; NHS, normal human serum.
Consistent with the well-established data on C3 fragment opsonization in patients with PNH treated with eculizumab,11,12,14,20,21 GPI-deficient erythrocytes opsonized with C3 fragments (ie, C3d+ GPI-deficient erythrocytes) were readily detected at baseline, with a mean value of 30% as a consequence of C3 fragment accumulation on cells having survived IVH in the presence of a C5 inhibitor.11,12 The addition of danicopan significantly decreased the percentage of GPI-deficient erythrocytes opsonized with C3 fragments (mean, 18% at week 24; Figure 4D). Concomitantly, the population of GPI-deficient erythrocytes increased from 54% at baseline to 84% (mean) at week 24, approaching the population of GPI-deficient granulocytes (mean, 92% at baseline and 95% at week 24; Figure 4D).
Safety
Danicopan was generally well tolerated. All patients reported ≥1 treatment-emergent adverse event (TEAEs). Most TEAEs were mild to moderate in severity; events reported in ≥2 patients included cough, headache, and nasopharyngitis (n = 3 patients each) and abdominal pain, arthralgia, fatigue, nausea, oropharyngeal pain, pain in extremity, and vaccination site pain (n = 2 patients each). Four patients had severe TEAEs. One patient had a nonserious severe (grade 3) episode of anemia that resolved; the investigator considered the event to be unrelated to danicopan, and the dose was not changed. One patient experienced a nonserious severe (grade 3) direct bilirubin increase that occurred in concert with a grade 1 increase in alanine aminotransferase (day 70). This patient had similar increases at baseline and during screening. The investigator considered these events unrelated to danicopan and believed that they were caused by breakthrough hemolysis because of the associated approximate doubling of LDH and the decrease in Hgb of 0.8 mg/dL. Both adverse events resolved (day 77). Danicopan treatment was not interrupted; the dose of danicopan was temporarily reduced and reescalated after the event resolved, and the patient completed the 24-week treatment period. There was no change to the eculizumab dose or dose interval.
A patient who had a history of neutropenia experienced a serious adverse event of pneumonia (day 145; evolving from viral bronchitis), required hospitalization, and recovered (day 152). This patient received a transfusion (2 units) during hospitalization. A relationship to danicopan was considered unlikely; the danicopan dose was not changed, and the patient completed the 24-week treatment period. One patient experienced a serious severe (grade 4) adverse event of worsening pulmonary hypertension/edema after 2 doses of study drug. This patient had preexisting pulmonary hypertension (valvular and as a consequence of PNH), and cardiac medications were changed a few days before study drug initiation. The event was considered unlikely related to danicopan; however, danicopan was withdrawn, and the patient withdrew from the study.
Discussion
C5 inhibition with eculizumab or ravulizumab is the standard of care for treatment of patients with PNH. Although this treatment inhibits the main cause of IVH and provides a dramatic improvement in overall survival, some patients remain anemic, and a subset may continue to be transfusion dependent because of persistent clinically evident EVH.11-14,22 Although ravulizumab can reduce the risk of breakthrough hemolysis compared with eculizumab,28,29 ravulizumab and eculizumab target C5 and do not address the residual anemia caused by EVH. In this trial, danicopan was added to eculizumab to investigate the clinical benefits of blocking EVH with a FD inhibitor in a subpopulation of patients who continued to require transfusions while on eculizumab therapy. The addition of danicopan resulted in a mean increase in Hgb of 2.4 g/dL and a clinically significant reduction in transfusion needs. The mean 11-point increase in FACIT-Fatigue score is also remarkable, because patients were receiving a stable dose of eculizumab at the start of the trial. Fatigue is often assessed with the FACIT-Fatigue scale in patients experiencing anemia, such as those with PNH.26,27 The International PNH Registry showed that fatigue is 1 of the most commonly reported symptoms in untreated patients, with ∼80% reporting fatigue 6 months prior to registry enrollment.27 Eculizumab monotherapy improved levels of fatigue in patients with PNH, as measured by FACIT-Fatigue, in the landmark SHEPHERD6 and TRIUMPH8 trials; scores increased significantly by 12.2 points and 6.4 points vs baseline, respectively. In the current trial, addition of danicopan in patients with transfusion-dependent anemia receiving eculizumab increased Hgb, as well as demonstrated a potential impact on patients’ quality of life. Specifically, addition of danicopan led to decreased patient-reported fatigue and a marked reduction in transfusion requirements (from 31 transfusions in 10 patients in the 24 weeks before initiating danicopan to 1 transfusion in a single patient during the 24-week primary treatment period). All patients who completed the primary treatment period elected to continue treatment in the long-term extension, further suggesting a potential positive patient impact.
There were meaningful improvements in bilirubin and reticulocytes, consistent with a reduction in EVH. Total bilirubin was decreased into the normal range at week 24 compared with baseline, and reticulocyte count was reduced to near normal at week 24. Additionally, the population of GPI-deficient erythrocytes approached that of GPI-deficient granulocytes, demonstrating that GPI-deficient erythrocytes were being further protected from hemolysis with the addition of danicopan.
The additional protection of GPI-deficient erythrocytes from hemolysis could be due to a reduction in IVH, EVH, or both. As described elsewhere, administration of danicopan monotherapy reduced LDH levels in untreated patients with PNH.25 However, it is not surprising that there was no impact on LDH in this study of danicopan add-on treatment; patients were on a stable regimen of eculizumab, and IVH was already well controlled. Hence, additional protection is most likely due to a reduction in EVH. Unlike danicopan monotherapy in which few C3 fragment–coated GPI-deficient erythrocytes were detected at baseline and during treatment, a readily detectable population of GPI-deficient erythrocytes coated with C3 fragments was found at baseline in this study, an observation that was reported in earlier studies of patients receiving eculizumab.11-13,21,30-32 In the current study, following the addition of danicopan to eculizumab, the percentage of GPI-deficient erythrocytes coated with C3 fragments was reduced, albeit with a rather large variability among patients. Incomplete abolishment of C3 fragment opsonization may reflect the PK and pharmacodynamics profile of danicopan, which, in some patients, does not result in a complete and sustained inhibition of AP activity. Alternatively, some patients may be refractory as a result of the presence of complement receptor 1 polymorphisms, which is a key determinant of the rate of C3 binding to GPI− RBCs.32 Nevertheless, the observed increases in Hgb and decreases in C3d+ GPI− RBCs, along with achievement of transfusion independence in 9 of 10 patients, suggest that AP inhibition by danicopan add-on confers a clinical benefit by reducing EVH and subsequent anemia. A second-generation FD inhibitor is being examined as monotherapy and as add-on to C5 inhibitors in an ongoing clinical trial (NCT04170023), which may address some of the PK limitations of danicopan.
Ravulizumab is a longer-acting C5 inhibitor engineered from eculizumab that has an extended half-life, requires less frequent dosing, and has been shown to be noninferior to eculizumab in phase 3 trials.11,28 Among those receiving ravulizumab in a phase 3 trial, 88% avoided transfusion compared with 83% of those receiving eculizumab; 76% of patients in each group achieved stabilized Hgb levels, indicating that a small subset of patients may still experience EVH and residual anemia, despite eculizumab or ravulizumab treatment.11,28 In the present study, addition of danicopan enhanced PNH management in patients who experienced EVH and residual anemia while receiving eculizumab, and it prevented transfusion for almost all patients. In the extension phase of this study (NCT03472885), patients receiving background eculizumab therapy are permitted to switch their C5 inhibitor to ravulizumab. Additionally, the ongoing randomized controlled phase 3 ALPHA trial (NCT04469465) is evaluating danicopan add-on to eculizumab or ravulizumab background therapy in patients receiving a C5 inhibitor and experiencing clinically evident EVH. The results of these studies will help the benefits and use of danicopan in addition to either C5 inhibitor.
Danicopan was generally well tolerated and resulted in meaningful improvements in Hgb, transfusion needs, FACIT-Fatigue score, and other parameters of interest when added to eculizumab in a subset of patients who were transfusion dependent. This demonstrates that further benefits can be achieved in patients receiving standard of care with C5 inhibitors by blocking the complement AP at the level of FD with danicopan. Danicopan has the potential to enhance PNH treatment and will be evaluated further in a pivotal trial in patients who remain anemic and are transfusion dependent, despite C5 inhibition.
Acknowledgments
The authors thank the patients and investigators, as well as their staff, who participated in this trial: Serena Marotta, Luana Marano, and Fabiana Cacace (Naples), Petra Muus and Shreyans Gandhi (London), Alberto Bosi and Federica Barone (Florence), Sung-Soo Park (Seoul), and Paul Hamilton (Auckland). In addition, they thank Heather Robison (Achillion Inc., a wholly-owned subsidiary of Alexion Pharmaceuticals, Inc.) and Steven Podos, Danny Shin, and Julia Catini (Alexion employees and former employees of Achillion Inc., a wholly-owned subsidiary of Alexion Pharmaceuticals, Inc.) for assistance with writing the manuscript and Peng Liu (Alexion employee) for statistical analysis support. Editorial assistance was provided by The Curry Rockefeller Group, LLC and was supported by Alexion Pharmaceuticals, Inc.
Authorship
Contribution: M.H. and M.G. developed the study protocol, with contributions by A.M.R., A.G.K., R.N., R.A.B., and P.B.; A.M.R., A.G.K., J.P.M., R.N., P.B., and R.A.B. recruited and treated patients and collected data; M.H. and M.G. analyzed and interpreted data and wrote the manuscript; and A.M.R., A.G.K., J.W.L., J.P.M., R.N., R.A.B., and P.B. contributed to the manuscript and approved its final version.
Conflict-of-interest disclosure: A.G.K. has served on advisory boards for Alexion, Celgene, Novartis, Ra Pharma, and Regeneron and has received travel grants from Achillion, Celgene, and Ra Pharma. A.M.R. has received research support from Alexion, Novartis, Alnylam, and Ra Pharma and lecture fees from Alexion, Novartis, Pfizer, and Apellis; has served on advisory/investigator boards for Achillion, Alexion, Apellis, BioCryst, Novartis, Roche, and Samsung; and has served as consultant for Amyndas. J.P.M. has received lecture honoraria from Alexion, Novartis, and Celgene and has served as a consultant for Novartis. R.N. has received lecture fees from Alexion and has served on the advisory board for BioCryst. P.B. has served on advisory boards for Merck, Janssen, Roche, and AbbVie and as an associate editor for the Internal Medicine Journal and has received research funding from Roche, BeiGene, and Amgen. J.W.L. has received grants from Alexion and Achillion; has served on advisory boards for Alexion and Apellis; has received honoraria from Alexion; and has served as a consultant for AlloVir. M.H. is an Alexion employee and has equity ownership in the company. M.G. was an employee of Achillion, a subsidiary of Alexion, and had equity ownership in the company. R.A.B has served on scientific advisory boards for Achillion and Alexion. The publication costs.
Correspondence: Robert A. Brodsky, Johns Hopkins University School of Medicine, Ross Research Building, Suite 1025, 720 Rutland Ave, Baltimore MD, 21205; e-mail: brodsro@jhmi.edu.
Presented in abstract form (interim results) at The New Era in Aplastic Anemia and Paroxysmal Nocturnal Hemoglobinuria Meeting, Naples, Italy, May 10, 2019; the 61st annual meeting of the American Society of Hematology, Orlando, FL, 9 December 2019; and the British Society for Haematology 2020 Virtual Annual Scientific Meeting, 9-14 November 2020.
Alexion supports transparency and disclosure and welcomes requests from qualified academic researchers for access to clinical data and applicable supporting documents pertaining to Alexion-sponsored interventional clinical studies considered in scope: https://alexion.com/our-research/research-and-development.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Inhibition of FD proteolytic activity by danicopan. FD participates in C3 convertase generation by cleavage of factor B at 2 steps in the AP cascade: generation of the initial C3 convertase [C3(H2O)Bb] following spontaneous AP activation (tick-over) in the fluid phase and the production of surface-bound C3 convertase (C3bBb), which mediates dramatic amplification of the initial activation (amplification loop) and activation of the terminal pathway (cleavage of C5 into C5a and C5b by C5 convertase [ie, C3bBb3b]), leading to opsonization of target surfaces by C3b, release of the anaphylatoxins C3a and C5a, and formation of MAC. An erythrocyte is shown to depict the membrane-bound events. Regulatory proteins not shown here can promote (properdin and FH-related proteins) or attenuate (factor H, factor I, multiple membrane-bound proteins, including CD55 and CD59) AP activity. Eculizumab acts directly at the terminal pathway by preventing its activation via binding to C5; danicopan acts upstream by preventing AP activation via inhibition of proteolytic activity of FD. Although the C5 inhibitor is able to block MAC formation regardless of the initiation pathway of complement activation, it has no effect on C3 fragment deposition. FD inhibitor is able to block C3 fragment deposition and MAC formation when the complement activation is initiated via AP, such as in the case of PNH; it should be able to exert the same, although not complete, inhibitory effect when complement activation is initiated via CP or LP through blocking the amplification loop.24](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/138/20/10.1182_blood.2021011388/7/m_bloodbld2021011388f1.png?Expires=1769111813&Signature=G5wisa7XgeQZmUbJ-DK4HdOeJCw1Yoyuk~-jQBt3-kTL80amLcgf9SLNXNl2AfHakvGURo16-hBJZ64017jUPCFF9DLd2m4mOvBqhtOvESb~dEt2x132H2ht8SxTxq7up5tl~Lqz97g4ePqHvmWFY4PQiuR~pp8pVdSpIsLA84WUfRGsWagyb7AqDfg4laX1cvzmf9Qh9RsnuHI3clUUletu4f3hEkck4L-yLmH5qvEvzoPqOn57Z3A7bFj-MsXLOSkr8IhLMU7dTM3rFhgLmlJW-tbowQRq5RJezG1srz0Yp~nPPeTXVxOmh-U4y~xN7RnHi1hSK6O9CNvv4O5Rnw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Effect of danicopan on laboratory markers. Mean (and standard deviation [SD]) change from baseline (BL) for Hgb (primary end point) (A), reticulocytes (B), total bilirubin (C), direct bilirubin (D), LDH (E), and FACIT-Fatigue score (F). P values represent change from baseline to week 24.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/138/20/10.1182_blood.2021011388/7/m_bloodbld2021011388f2.png?Expires=1769111813&Signature=wFSfAdkwSBM6EbB8UoMOVM0VQvYv06HUpF2sdhHh7mBJYqTaJ1k1kiT3~moy-hsGx3q8SwC2jXHQ4MUAbGel6U4TtPBnOarvE4vbpiRzrcNY9LR93QlaJ9X0lNySs~4B-bwKhKsS3EqLLAPU1UsDcos-aIU2lQxaCFNCQfpueE5XDJx-jw1DALO~NcokWhyA2yDuiBnV01GF1pU7f69qVObwkeLmMcDzRfsaZHZgFNXOLlszzdvti1U0sOc542Huf2V7MyIpUOK107OxczSZmTfI2BORoEnvzf-6s60DhUCDoRBKNKp~4v8HtrIQOmZshivNUQwGoE3sw8RNQrLxyg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Effect of danicopan on complement biomarkers and GPI-deficient cell population. Serum, plasma, and whole blood samples were collected at day 1 prior to dosing danicopan (baseline [BL]) and at the indicated time points during the study and subjected to measurement of CP activity (A), AP activity with AP hemolysis assay (B), plasma Bb concentration (C), and the population size of GPI-deficient granulocytes, GPI-deficient erythrocytes, and C3d+ GPI-deficient erythrocytes (D), as described in Methods. Arithmetic mean and standard derivation (SD) are shown for all, with the range shown at each time point. LLN, lower limit of normal; NHS, normal human serum.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/138/20/10.1182_blood.2021011388/7/m_bloodbld2021011388f4.png?Expires=1769111813&Signature=4FpXWJ0hWr90rgpvG79o0FWPtqixR7gLHPsj~UU0pZCTTBnHxIlAjNQWcM3lFNpMdsCu9eiN1OVif74sR1ihqDzP~JEweaAs4oKE9Jkj1~F5SG50BQ57HANPpcI13OHAreo9CaiQpvZuRT-7KE4NMcTNCvZWEf1WcFQxFfQemuc7jKIp0kdUf5LlVMCetDfIjWGchhRUJiMDHXauPBP2xpeSH-4sC6Q-LPeGPl2-kxW~qP~ygkFRxdJc1R8g8eNthnEOemqZd0yT38IqN~vK0UPltLT5Q5SihQFyBKVCRkKpNgvB93gwUtam0hIhW8jdPheaixulDBZ2cYE2DAaduQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal