The outlook for patients with chronic phase chronic myelogenous leukemia (CML) has improved dramatically since the development in the late 1990s of oral tyrosine kinase inhibitors (TKIs) targeting the ATP binding site of the BCR-ABL1 oncoprotein. In this issue of Blood, Réa et al1 address the management of patients in chronic phase previously treated with ≥2 TKIs in a randomized trial comparing asciminib and bosutinib, which is approved for use in this patient population. Ascinimib (previously known as ABL001) is a small molecule which binds to the myristoyl pocket located at a different site on the BCR-ABL1 protein, producing a conformational change that inhibits downstream signaling.
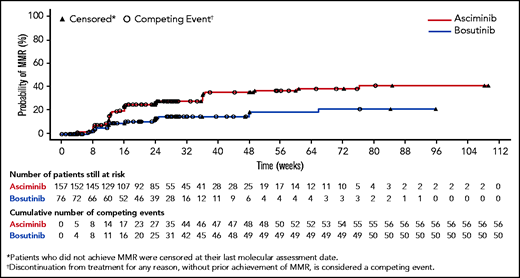
The cumulative incidence of major molecular response (MMR) by week 24, the primary study endpoint, was 25% with asciminib vs 12% with bosutinib (P = .029) with corresponding complete cytogenetic response rates of 41% and 24.2% (see figure). This response rate with asciminib is consistent with that seen in the original phase 1 study,2 and the overall side-effect profile compared favorably to the bosutinib arm. Follow-up is short (see figure), and the duration of response cannot be assessed.
Despite the randomization, virtually all prerandomization adverse risk factors, including the number intolerant compared with resistant to their prior TKI, the number of prior lines of treatment, and the number of BCR-ABL1 kinase mutations, were slightly imbalanced in favor of the asciminib arm. Nevertheless, the responses noted in both asciminib trials are of clinical benefit to this subset of patients and validate the approach of blocking the signaling effect of the myristoyl site. However, the response rates are perhaps lower than what might have been hoped for, suggesting that ATP or myristoyl pocket signaling might still be present and/or that asciminib has little effect on the other unknown non-mutation-driven pathways leading to TKI resistance. The lower response rate is also a reminder that patients with resistance to multiple TKIs present a serious clinical problem with many such individuals destined for allogeneic transplantation.
How might these findings influence the future management of chronic phase CML? To provide perspective:
- •
The survival of patients in chronic phase responding to TKIs approximates that of age-matched controls with remarkably similar results recently reported from less medically developed countries supplied with imatinib from the charitable MAX Foundation.3
- •
Although initial treatment with nilotinib, dasatinib, or bosutinib produces more rapid and deeper molecular responses than imatinib, overall survival is identical in randomized trials comparing imatinib with these agents.4,5
- •
Approximately 30% to 40% of patients started on any TKI switch to an alternative TKI because of side effects, or less often, inadequate response. Although persistent low-grade, but nonetheless bothersome side effects, such as fatigue, muscle cramps, and diarrhea, occur with imatinib, there are few, if any, long-term effects on organ function, whereas an increase in thrombotic cardiovascular events is seen with other TKIs, particularly nilotinib.5
- •
Generic versions of imatinib have increased access to treatment worldwide and decreased the annual cost in the United States to <$5000, compared with the previous obscene pricing of >$10 000 per month, which seemed to set the bar for pricing of most oral targeted anticancer agents in the United States.
- •
Multiple studies have shown that ∼50% of patients who have maintained deep molecular responses can safely discontinue treatment without molecular relapse (treatment free remission, TFR).6 The rates of TFR are similar using imatinib, nilotinib,7 or dasatinib8 as initial treatment. TFR has become a major goal of treatment, but rough estimates extrapolated from the results of clinical trials suggest that only ∼15% to 25% of newly diagnosed patients might eventually achieve TFR, albeit after many years of TKI treatment.6
Given this background, how might asciminib improve matters and, in particular, might earlier use increase the rate of successful TFR with less toxicity?
There are a relatively modest number of active clinical trials using asciminib listed in clinicaltrials.gov, with a few focused on Philadelphia chromosome–positive acute lymphoblastic leukemia as well as pilot studies in combination with imatinib, dasatinib, and nilotinib. It is not obvious that “hitting” the same gene product twice with combination treatment will produce deeper responses or elimination of stem cells, although it is reasonable to address this question in future trials. Indeed, the CML stem cell, which persists in a dormant state when suppressed by TKI therapy, is remarkably hardy, as evidenced by rapid molecular relapses after TKI discontinuation, even in patients with nondetectable transcripts who had been on therapy for years.
The cumulative incidence of MMR for the bosutinib and asciminib arms is shown. See Figure 3 in the article by Réa et al that begins on page 2031.
The cumulative incidence of MMR for the bosutinib and asciminib arms is shown. See Figure 3 in the article by Réa et al that begins on page 2031.
At least 3 patients in the ASCEMBL trial developed mutations in the myristoyl binding pocket leading to resistance.1 In vitro experiments suggest that combination therapy may decrease the development of such mutations in the ATP and myristoyl sites.9 However, mutations modifying the ATP binding site are detected in at most half of patients resistant to TKIs, and the results with asciminib suggest that there is incomplete activity against other poorly characterized mechanisms of resistance.
Other studies propose adding asciminib in patients who have not achieved deep molecular responses with TKIs to see if such patients can attain responses sufficient for a trial of discontinuation. It is notable, however, that an investigation of similar design evaluating the substitution of nilotinib compared with continued imatinib was not successful and was accompanied by cardiovascular events.10
The pathophysiology of the nonhematopoietic toxicities of TKIs is poorly understood but has been attributed to “off-target” effects mediated by other tyrosine kinases. It is of interest that the putatively more “targeted” asciminib also has hematopoietic and systemic side effects, and in particular, a pattern of elevated lipase as well as occasional frank pancreatitis.2 The long-term tolerance is not known.
Thus, the asciminib trials clearly confirm preclinical observations that targeting the myristoyl site of the BCR/ABL1 can be of value to some patients whose CML is resistant to standard TKI therapies. The pace of clinical development has been a bit slow, and the hope is that clinical trials will begin in earlier stages of treatment with the long-term goal of improving the rate of TFR, acknowledging, however, the complexity of such trials.
Conflict-of-interest disclosure: The author has served on data safety and monitoring boards for BMS/Celgene, Astellas, Syndax, and Kartos; and as consultant for or on advisory boards for Agios, Merck, BMS, and Novartis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal