In this issue of Blood, Henderson et al1 identify plasmin cleavage of high-molecular-weight kininogen (HK) as a novel mechanism mediating acetaminophen-induced hepatotoxicity.
The contact system, also named as the plasma kallikrein-kinin system, involves 3 serine proteinases: coagulation factors XII (FXII) and XI (FXI) and plasma prekallikrein (PK), as well as the nonenzymatic cofactor HK. This system is a plasma protease cascade initiated by activation of FXII, which then triggers the intrinsic coagulation pathway, stimulates proinflammatory response, and generates vasodilator bradykinin.2,3 Although this system has been actively investigated for more than 6 decades, its physiologic and pathophysiologic role still remains largely unknown. Now, as reported by Henderson et al, the cleavage of HK by plasmin plays a critical role in acetaminophen-induced liver injury (AILI). This study reveals a novel function for the contact system component in drug-induced systemic inflammation and tissue damage.
Acetaminophen overdose–induced liver injury is the leading cause of drug-induced liver failure in the Western countries. It has been known that acetaminophen induces activation of coagulation and fibrinolysis, which contributes to AILI; however, the mechanism is still unclear. The authors of the current study found that targeted ablation of HK mitigates AILI. By screening upstream regulators, they found that the deficiency of FXII, FXI, or PK has no protection against AILI, demonstrating that HK, but not other components of the contact system, is exclusively crucial in hepatocyte damage. This new observation is consistent with previous studies showing that HK has unique functions that are independent of the contact system activation.2-4 As HK deficiency does not inhibit coagulation activation but does ameliorate acetaminophen-induced production of the circulating cytokines interleukin 6 (IL-6), IL-1β, macrophage inflammatory protein (MIP-1β), and granulocyte colony-stimulating factor (GCSF). Thus, the role of HK in AILI is proinflammatory and not related to coagulation. Because the reconstitution of HK−/− mice with human HK protein restores AILI, HK may have the same function in human subjects. Indeed, in plasma from patients with AILI, enhanced HK cleavage and plasmin generation were observed, suggesting that HK is cleaved by other proteases such as plasmin. In a purified system and plasma, plasmin indeed cleaves HK, with the subsequent release of bradykinin and other HK fragments (60 and 52 kDa) in a manner independent from the contact system. Furthermore, they found that plasminogen deficiency attenuated AILI and prevented HK cleavage. However, the deficiency or antagonists of the bradykinin receptor has no protective effect, indicating that HK fragments other than bradykinin contribute to acetaminophen-induced inflammation. These observations provide solid evidence that plasmin cleavage of HK constitutes a novel pathway mediating AILI.
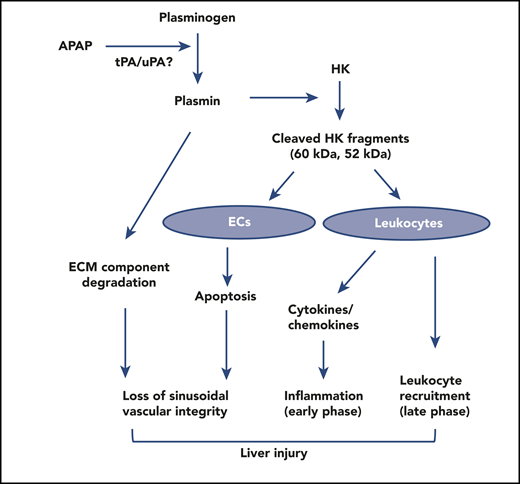
Possible mechanisms underlying the role of plasmin and HK cleavage in AILI. Acetaminophen overdose induces plasmin generation by tissue plasminogen activator (tPA) or urokinase plasminogen activator (uPA), which degrades the extracellular matrix (ECM) component fibronectin and cleaves HK. Cleaved HK induces apoptosis of endothelial cells (ECs) and inhibits angiogenesis. Thus, both plasmin and cleaved HK could disrupt sinusoidal vascular integrity and repair. Cleaved HK stimulates leukocytes to release inflammatory and chemotactic cytokines, inducing systemic and local inflammation and regulating neutrophil recruitment in the resolution phase. Through these multiple pathways, plasma and HK cleavage mediates AILI. APAP, N-acetyl-para-aminophenol.
Possible mechanisms underlying the role of plasmin and HK cleavage in AILI. Acetaminophen overdose induces plasmin generation by tissue plasminogen activator (tPA) or urokinase plasminogen activator (uPA), which degrades the extracellular matrix (ECM) component fibronectin and cleaves HK. Cleaved HK induces apoptosis of endothelial cells (ECs) and inhibits angiogenesis. Thus, both plasmin and cleaved HK could disrupt sinusoidal vascular integrity and repair. Cleaved HK stimulates leukocytes to release inflammatory and chemotactic cytokines, inducing systemic and local inflammation and regulating neutrophil recruitment in the resolution phase. Through these multiple pathways, plasma and HK cleavage mediates AILI. APAP, N-acetyl-para-aminophenol.
There are a multitude of possible mechanisms underlying the role of plasmin in AILI (see figure). First, acetaminophen overdose induces plasmin generation, which degrades the extracellular matrix component fibronectin, disrupting hepatic sinusoidal vascular integrity.5 The cleaved fragments of HK containing domain 5 (D5), but not bradykinin, releases the cytokines tumor necrosis factor-α, IL-6, and IL-1β, nd chemokines IL-8 and MCP-1 from mononuclear cells, which may induce systemic and local inflammation.6 Because HK−/− mice have significantly less circulating inflammatory and chemotactic cytokines IL-6, IL-1β, MIP-1β, and GCSF, HK regulates inflammatory response and neutrophil recruitment in AILI. Recruitment of neutrophils to liver is involved in the resolution phases of inflammation.7 D5 is antiadhesive and decreases the recruitment of neutrophils,8 and the fragments with D5 may delay the resolution of acetaminophen-induced hepatocyte injury, consistent with a significant reduction in the number of infiltrating neutrophils in the livers of HK−/− mice 24 hours after acetaminophen overdose. Furthermore, D5 induces apoptosis of endothelial cells and inhibits angiogenesis9,10 ; thus, it may disrupt barrier function and affect vascular remodeling. Thus, it is very likely that the pathologic effects of plasmin–HK cleavage in AILI is mediated by multiple and nonoverlapping pathways.
This intriguing study raises additional questions for future investigation. First, the mechanisms by which acetaminophen overdose induces plasmin activation are not clear and need to be further explored. Moreover, plasmin-cleaved specific fragments of HK (molecular weight ∼60 kDa, molecular weight ∼52 kDa?) that are responsible for AILI awaits to be determined. Low-molecular-weight kininogen (LK) in plasma shares the same cleavage sites with HK-releasing bradykinin; does LK also contribute to acetaminophen hepatotoxicity? To prove the alternative hypothesis that the cleavage of HK is simply the presence and not necessary for mediating injury severity in this model, HK cleavage-resistant mutant could be used to test this hypothesis. In addition to plasmin, other enzymes, such as neutrophil protease 3, cathepsins, calpain, mannose-binding lectin-associated serine protease-1, and elastase, can cleave HK.2,3 Additional studies with deletion of these genes may determine their implication and further clarify HK as a general mediator in AILI. Finally, characterization of cleaved HK in human patients with AILI will improve the evaluation of HK-derived inflammation in this scenario. In future studies, we will need to connect the dots and demonstrate that the cleavage of HK is critical, determine the cleavage sites of HK by plasmin, and show that the inability to cleave HK interferes with the process of AILI. The studies by Henderson et al et al lay a solid groundwork and pave the way toward these goals. A better understanding of the mechanisms how acetaminophen overdose induces the activation of plasmin and the cleavage of HK in AILI is necessary for the development of novel antidotes.
In summary, Henderson et al provide persuasive data supporting the pivotal role of cleaved HK in liver injury challenged by acetaminophen overdose. They demonstrate that plasmin, not the contact system proteases, cleaves HK, forming a new pathway that contributes to AILI. This work adds HK to the list of downstream effectors of plasmin activation that exerts multiple roles in AILI. As the animal models with the deficiency of both plasminogen and HK indicate the protective effect in AILI, targeting this plasmin-HK pathway has therapeutical potential in AILI. Inspired by the current study, further evaluation of the plasmin-HK cleavage pathway in other forms of tissue damage will improve our knowledge of the pathophysiology of the contact system.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal