Abstract
The development of vaccines to fight COVID-19 has been a remarkable medical achievement. However, this global immunization effort has been complicated by a rare vaccine-related outcome characterized by thrombocytopenia and thrombosis in association with platelet-activating anti–platelet factor 4 antibodies. In this Spotlight, we will discuss the recently described complication of vaccine-induced immune thrombotic thrombocytopenia (VITT) occurring in response to certain COVID-19 vaccines. Although information about this clinical condition is rapidly evolving, we will summarize our current understanding of VITT.
Introduction
The global effort to curb the spread of the COVID-19 infection has been remarkable for its speed and efficacy. Within 1 year of the arrival of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on the world stage, pharmaceutical companies developed and delivered vaccines that have dramatically reduced the burden of COVID-19 disease. In the United Kingdom, 1 dose of a commercially approved vaccine effectively reduced hospitalization and death by >80%1 ; in the United States, hospitalization rates from COVID-19 were reduced by 67% and 94% with single and dual vaccination, respectively.2
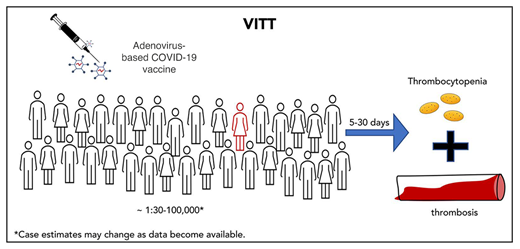
In late February 2021, initial descriptions of a safety signal emerged with the adenoviral-based (ChAdOx1-S) COVID-19 vaccine distributed by AstraZeneca (AZ).3,4 These reports detailed otherwise healthy individuals developing complications of thrombocytopenia and thrombosis in atypical locations (cerebral and/or splanchnic veins) within weeks of receiving the vaccination. By mid-April 2021, similar complications were described with another adenoviral-based vector (recombinant Ad26.COV2.S) distributed by Johnson & Johnson (J&J).5,6 This syndrome has been variably referred to as vaccine-induced immune thrombotic thrombocytopenia (VITT) or thrombosis with thrombocytopenia syndrome. For this article, we will use the more specific term VITT, given the temporal relationship of disease with COVID-19 vaccination.
Although descriptions of VITT are recent (<3 months as of this publication), published case reports/case series are relatively few, and research is limited, there is coalescing knowledge about the epidemiology, pathogenesis, diagnosis, and management of this syndrome. In this article, we will review what is currently known and unknown about VITT.
VITT is a vaccine safety signal with adenoviral-based vectors
We know that VITT is a safety signal from adenoviral-based vectors. What made this safety signal discernible was the concurrent presentation of thrombocytopenia and thrombosis, a rare occurrence in the general population and those immunized with nonadenoviral-based SARS-CoV-2 vaccines.
As of 12 May 2021, the estimated incidence of VITT was ∽7 to 10 cases per million individuals with the AZ vaccine (∽309 cases reported out of 32.9 million doses given in the United Kingdom)7 and ∽3.2 cases per million for the J&J vaccine (or 28 cases out of 8.7 million doses administered in the United States)5 (Table 1). The reported rates for the J&J vaccine likely underestimate the true incidence of disease, given the shorter period of J&J vaccine availability (emergency use authorization was granted in the United States on 27 February 2021) and delays in reporting of the syndrome.
Clinical features of VITT associated with AZ and J&J vaccines
| Features . | UK cases7 . | US cases5 . |
|---|---|---|
| Total no. of cases | 309 | 28 |
| Total no. of vaccines administered, million | 32.9 | 8.7 |
| Reporting rate, per million | 9.4 | 3.2 |
| No. after first-dose vaccine (%) | 294 (95) | 28 (100) |
| Female (%) | 169 (55) | 22 (78) |
| Age range, y | 18-93 | 18-59 |
| No. of patients <50 y (%) | 129 (42) | 22 (78) |
| No. of patients <70 y (%) | 254 (82) | 28 (100) |
| CVT (%) | 116 (38) | 19 (68) |
| ICH (%) | NR | 10 (36) |
| Death (%) | 56 (18) | 3 (11) |
| Features . | UK cases7 . | US cases5 . |
|---|---|---|
| Total no. of cases | 309 | 28 |
| Total no. of vaccines administered, million | 32.9 | 8.7 |
| Reporting rate, per million | 9.4 | 3.2 |
| No. after first-dose vaccine (%) | 294 (95) | 28 (100) |
| Female (%) | 169 (55) | 22 (78) |
| Age range, y | 18-93 | 18-59 |
| No. of patients <50 y (%) | 129 (42) | 22 (78) |
| No. of patients <70 y (%) | 254 (82) | 28 (100) |
| CVT (%) | 116 (38) | 19 (68) |
| ICH (%) | NR | 10 (36) |
| Death (%) | 56 (18) | 3 (11) |
CVT, cerebral vein thrombosis; ICH, intracerebral hemorrhage; NR, not reported; UK, United Kingdom; US, United States.
The epidemiology of VITT must be considered in the context of similar complications in the general population. The annual incidence of isolated thrombocytopenia, such as immune thrombocytopenia (ITP), or isolated cerebral vein thrombosis (CVT) is higher than that reported for VITT, but when adjusted for the 2-week time frame characteristic of VITT presentations, corresponding rates are lower than that of VITT. ITP in the general population occurs in 16 to 39 cases per million (or 0.61-1.5 cases per million in any 2-week period),8,9 whereas isolated CVT is reported in 13 to 20 cases per million (0.5-0.77 cases per million in any 2-week period).10,11 Although there are no formal studies of thrombocytopenia in association with CVT, other well-known thrombotic thrombocytopenic syndromes, such as thrombotic thrombocytopenic purpura and/or atypical hemolytic uremic syndrome, occur at a frequency of 11.3 cases per million (or 0.43 cases per million in any 2-week period).
Hematologic complications of either isolated ITP or CVT occurring in the wake of COVID-19 vaccination also appear to be lower than reported rates of VITT. Complications of ITP for the 2 messenger RNA (mRNA)-based vaccines have been estimated to occur in 0.8 to 1 case per million, based on reporting to the Vaccine Adverse Event Reporting System (VAERS),12 whereas isolated CVT after vaccination with BioNTech/Pfizer is ∽4 cases per million (2 of 489871).13
There is also persuasive epidemiologic data on VITT to suggest a class effect among vaccines. The 2 major types of vaccine technologies that have been approved in the United States and Europe include formulations of lipid nanoparticles containing mRNA encoding the SARS-CoV-2 spike protein (BioNTech/Pfizer and Moderna) or replication-defective adenoviral vectors (AZ and J&J) expressing modified DNA for the SARS-CoV-2 spike protein. The AZ vaccine uses a chimpanzee adenoviral vector (ChAdOx1-S) that encodes a modified membrane-bound SARS-CoV-2 spike protein that does not shed, whereas the J&J vaccine uses a recombinant human adenovirus type 26 vector (Ad26.COV2.S) encoding an unmodified spike glycoprotein. To date, of the >200 million doses of mRNA-based vaccines administered in the United States, there have been no documented reports of thrombosis complicated by thrombocytopenia.5
VITT is pathogenically linked to autoimmune heparin-induced thrombocytopenia
We have preliminary insights into VITT pathogenesis. Published reports indicate that VITT is: (1) an immune complication resembling a variant of autoimmune heparin-induced thrombocytopenia (aHIT), (2) unlikely a byproduct of COVID-19 infection, and (3) independent of anti–SARS-CoV2 protective immunity.
Demonstration of circulating anti–platelet factor 4 (anti-PF4) antibodies in conjunction with thrombocytopenia and thrombosis suggests that VITT is a clinical variant of aHIT.3-6,14,15 Anti-PF4 antibodies are the hallmark of heparin-induced thrombocytopenia (HIT), a thrombotic disorder caused by the anticoagulant drug heparin. Spontaneous HIT, a rare manifestation of HIT, occurs without prior heparin exposure and, in most cases, is precipitated by recent infection and/or orthopedic surgery.16 Although disease manifestations in HIT are caused by antibodies directed to ultralarge complexes of PF4 bound to heparin or polyanions, such as glycosaminoglycans, polyphosphates, or DNA,17-21 aHIT is associated with anti-PF4 antibodies whose functional effects are largely independent of heparin or polyanions.16 Pathogenic anti-PF4/heparin antibodies cross-link FcγRIIA on platelets, monocytes, and neutrophils to initiate procoagulant cellular responses that generate a profound hypercoagulable state.22 Thrombotic risk in HIT and/or aHIT is strongly correlated with high levels of circulating anti-PF4 antibodies, as detected by immunoassays and/or functional assays of platelet activation.23-25
It is also clear from recent data that VITT is not an aberrant clinical manifestation of COVID-19 infection. Although COVID-19 is recognized as a hypercoagulable disorder, complications of CVT with or without thrombocytopenia are uncommon in the wake of infection. In a recent study of ∽500 000 patients with documented COVID-19, 20 patients developed CVT (0.004%), whereas thrombocytopenia with CVT was noted in only 1 patient.13 Additionally, most VITT patients do not have active COVID-19 infection as documented by negative SARS-CoV-2 RNA testing at time of disease presentation.3,4,14,15,26
The immune responses to SARS-CoV-2 proteins and PF4, as seen in COVID-19 and VITT patients, respectively, are also nonoverlapping. Although high levels of anti-PF4 antibodies are present in nearly all patients with VITT,3,4,6,14,15,26 anti-PF4 antibodies are found at the expected prevalence (∽8% to 12%) in hospitalized patients with COVID-19, who are likely exposed to heparin during treatment.27,28 There are also no significant differences in the incidence of anti-PF4 antibodies in COVID-19 patients with and without thrombosis to support a pathogenic role for anti-PF4 antibodies.27 Finally, serologic studies do not show antigen cross-reactivity between anti–SARS-CoV-2 and anti-PF4 antibodies, signifying that anti-PF4 antibodies are unlikely a byproduct of anti-SARS-CoV-2 immunity.27
VITT is a clinically distinct syndrome
We recognize that VITT is a clinically distinctive syndrome with (1) a propensity for cerebral and/or splanchnic vein thromboses, (2) laboratory features showing consumptive coagulopathy in association with anti-PF4 seropositivity, and (3) poor outcomes.
To date, the largest numbers of cases with VITT seen in association with the AZ (n = 242) and J&J (n = 15) vaccines have been reported by the Medicines and Healthcare Products Regulatory Agency in the United Kingdom and the Centers for Disease Control and Prevention (CDC) in the United States, respectively (Table 1).5,7 Although initial reports indicated disease predisposition in younger female patients,3,4,14,15 a larger data set from the United Kingdom7 suggests a more modest age and sex imbalance. Disease incidence appears to be lower among individuals over the age of 70 years (18% with AZ and none with J&J) with a slight female predominance (1.4:1, female to male for AZ; see Table 1). Published case series do not show any consistent association with medical comorbidities, including use of oral contraceptive pills, hormonal therapy, cardiovascular disease, or thrombophilia.3,4,14,15 The majority of subjects present after 1 dose of vaccine, often within weeks of immunization.3-5,7,14,15 Presenting symptoms are reflective of underlying thrombosis and include headaches, nausea, vomiting, and/or abdominal pain. CVT is the most common site of thrombosis in reported series and occurs in 38% to 80% of reported cases,3-7,14,15 followed by involvement of the splanchnic bed (portal, spleen, and/or mesenteric veins). These atypical sites of thrombosis in VITT stand in distinction to those in HIT and aHIT, where thromboses occur in more typical locations, such as deep venous thrombosis, pulmonary embolism, and/or arterial thromboses.29-32
The laboratory features of VITT indicate a consumptive coagulopathy (thrombocytopenia, low fibrinogen, and elevated D-dimer), which is only seen in the most severe cases of HIT.33 When performed, tests for acquired thrombophilias such as anti-phospholipid antibodies, paroxysmal nocturnal hemoglobinuria, ADAMTS13 deficiency, and myeloproliferative neoplasms are usually normal.3-5,7,14,15 Circulating anti-PF4/heparin antibodies are detectable in most patients using enzyme-linked immunosorbent assays (ELISAs) but not latex immunoturbidometric assays, the latter of which is a technique that relies on competitive inhibition with latex particles coated with a monoclonal HIT-like monoclonal antibody.14,15,34 Although most reports demonstrate a strong correlation of anti-PF4 seropositivity with functional assays of platelet activation,3,4,14 1 recent case series of J&J vaccine subjects indicated low rates of positivity in functional assays (only 1 of 9 VITT subjects had platelet-activating antibodies).15 Clinicians should be aware that functional studies may yield false-negative results in VITT, as standard platelet-activation assays for detection of HITT antibodies, such as the serotonin release assay, rely on low (0.1-1 U/mL) and high (10-100 U/mL) doses of heparin to demonstrate heparin-dependent activity. VITT antibodies show PF4-dependent activity and may lose reactivity in the presence of heparin.27 Ideally, functional assays for VITT antibodies should be performed in the presence or absence of added PF4.
VITT is a morbid complication associated with high fatality rates. Where reported, cerebral hemorrhage complicates thrombosis in 26% to 80% of published case series3,4,14,15 and is the leading cause of death. Case fatality in patients with VITT is ∽20% (Table 1), likely due to delayed recognition of clinical symptoms and signs by affected individuals and/or providers. It is too early to understand the long-term outcomes in those recovering from CVT and/or splanchnic vein thromboses.
VITT should be managed as a HIT-like syndrome
We have sufficient evidence in the form of shared clinical and laboratory features between VITT and aHIT to approach the management of VITT as a HIT-like syndrome. In the published case series, treatment with unfractionated heparin or low-molecular-weight heparin and/or platelet transfusions may have contributed to disease progression,4,14 whereas treatment with nonheparin anticoagulants and IV immunoglobulin (IVIG) was often associated with recovery.
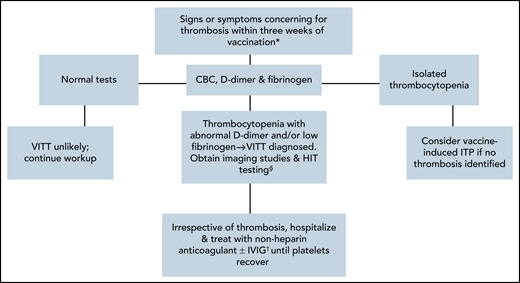
Based on these reports and established protocols for treatment of HIT, we recommend the following approach to the management of VITT patients (Figure 1). For recently vaccinated patients (within 4-30 days) presenting with new symptoms (headaches, visual changes, nausea, vomiting, abdominal pain, shortness of breath, chest pain, etc), we advise initial testing with a complete blood count, D-dimer, and fibrinogen, as abnormalities would identify patients with severe manifestations of the syndrome. If all of these laboratory tests are normal, then VITT is less likely; however, patients should be followed, and laboratory testing repeated should symptoms persist. If there is only isolated thrombocytopenia, with normal D-dimer and fibrinogen, and no thrombosis, a diagnosis of vaccine-induced ITP12,35 should be considered, particularly if the patient had received 1 of the mRNA-based vaccines. If there is evidence of consumptive coagulopathy as indicated by decreased platelets, elevated D-dimer, and/or low fibrinogen, we advise obtaining imaging studies and an anti-PF4 ELISA for documentation of VITT.
Diagnostic algorithm for patients suspected of VITT. Recommended diagnostic algorithm for patient presenting with signs and symptoms of VITT. *Symptoms may include: headache, visual disturbances, fever, hemiparesis, abdominal pain, dyspnea, and limb swelling/pain. §Anti-PF4 ELISA testing plus serotonin-release assay for confirmation of VITT diagnosis; if studies are negative, but clinical suspicion high, avoid heparin products. †IVIG as sole therapy for thrombosis should be considered only if anticoagulation contraindicated.
Diagnostic algorithm for patients suspected of VITT. Recommended diagnostic algorithm for patient presenting with signs and symptoms of VITT. *Symptoms may include: headache, visual disturbances, fever, hemiparesis, abdominal pain, dyspnea, and limb swelling/pain. §Anti-PF4 ELISA testing plus serotonin-release assay for confirmation of VITT diagnosis; if studies are negative, but clinical suspicion high, avoid heparin products. †IVIG as sole therapy for thrombosis should be considered only if anticoagulation contraindicated.
If imaging studies reveal thrombosis, we recommend hospitalization, initiation of treatment with nonheparin anticoagulants and avoidance of platelet transfusions. A significant proportion of these patients also have intracerebral hemorrhage, however, which can complicate initial management. Although current neurosurgical guidelines recommend anticoagulation in those with intracranial hemorrhage,36,37 anticoagulation decisions in this setting should be individualized and occur in collaboration with neurological or neurosurgical specialists. Adjunctive therapies for management of severe VITT thromboses include treatments used for refractory HITT, such as IVIG,38,39 plasma exchange,40,41 and mechanical thrombectomy.42,43 For bleeding complications, we suggest IVIG and/or prednisone to improve platelet counts and cryoprecipitate and/or fresh frozen plasma for correction of coagulopathy. Case reports also indicate that patients may present with thrombocytopenia, coagulopathy, and anti-PF4 antibody positivity, but without thrombosis,44 in which case, patients should be managed similarly as patients with thrombosis using nonheparin anticoagulants until platelet counts and coagulopathy resolve. Once patients recover, we recommend continuing oral anticoagulation for a minimum of 3 months.
Many unknowns remain about VITT
We concede that the knowledge base informing this review is currently limited; emerging data may shift our current understanding and there remain many more unknowns than knowns. We still do not have clarity on the incidence of disease and impact of age, sex, and race, as surveillance systems are passive, and many cases are likely unreported. Whether the class effects and differences seen in VITT incidence associated with AZ and J&J vaccines are related to the DNA cargo, contaminants, or the adenovirus vector itself is also unknown at this time. A number of questions remain about PF4’s causative role in the immune response and identification of biomarkers and/or genetic susceptibility that could portend thrombotic risk. Finally, there remain uncertainties regarding management and long-term outcomes: how long should patients be treated with anticoagulation, are future adenoviral-based vaccines or therapies contraindicated, and should VITT patients avoid heparin in their future?
Despite these unknowns, our knowledge of this disease has progressed at a remarkable pace. With increased disease awareness and recognition of this syndrome by providers and the public alike, future cases should help refine our clinical understanding and improve clinical outcomes.
Authorship
Contribution: G.M.A. and T.L.O. wrote and reviewed the manuscript.
Conflict-of-interest disclosure: G.M.A. serves as a consultant for AstraZeneca and Novartis. T.L.O. declares no competing financial interests.
Correspondence: Gowthami M. Arepally, Division of Hematology, Duke University Medical Center, Box 3486, Room 356A Alex H. Sands Bldg, Research Dr, Durham, NC 27710; e-mail: arepa001@mc.duke.edu.

Comments
Pathological mechanism in vaccine-induced thrombotic thrombocytopenia (VITT) of adenoviral-based anti-COVID-19 vaccines
Arepally, et al. demonstrated the crucial role of PF4 antibodies in the VITT process[1]. But the authors did not adequately discuss the pathogenesis. Herein, we propose one of the important pathological mechanisms in VITT: The negatively charged proteins along with the S protein produced by adenovirus vaccine, such as adenovirus skeleton protein.
Recently,Almuqrin, et al. [2] has found that the genome of ChAdOx1 may express low-level adenovirus skeleton protein, 28 kbp of adenovirus genes are delivered to the cell nucleus alongside the COVID-19 S glycoprotein gene in some cells lines such as A549 and MRC5, the expression of adenovirus cytoskeleton protein can be detected. Besides, McGonagle, et al. [3] has also pointed out that a role for adenoviral proteins has been suggested as a potential factor in VITT in susceptible individuals. The susceptible individuals, may be those people who could express adenovirus skeleton protein and reach a certain high titer[4].
The possible routes for the formation of PF4 antibodies:
(1) The extravascular route: after the injection,the adenovirus enters into the muscle cell, expressing the spike protein mainly, but for some susceptible individuals, the adenovirus gene is also expressed. And if local microbleeding accident happened, the adenovirus vector proteins bind with the PF4, then uptaken by APC cells, what happens in the regional lymph-nodes, secreting PF4 autoantibody which would trigger the VITT process through lymphatic circulation.
(2) The intravascular route: after the injection, the adenovirus leak into the blood circulation, travel to distant and different tissues and infect a range of permissive cells, which means susceptible, secreting the adenovirus vector proteins, then the adenovirus vector proteins bind with PF4, and trigger the VITT process as above.
Route 1 takes relatively short time while route 2 takes relatively long time, which is accordant with clinical situation[5].
Declaration of Competing Interest
The authors declare no competing interests.
Contributor Information
Yimin Cui, Email: cui.pharm@pkufh.com
Reference
[1] Arepally G.M, Ortel T.L. Vaccine-induced immune thrombotic thrombocytopenia: what we know and do not know. Blood. 2021; 138(4): 293–298.
[2] Almuqrin A, Davidson A.D, Williamson M.K, Lewis PA, Heesom KJ, Morris S, et al. SARS-CoV-2 vaccine ChAdOx1 nCoV-19 infection of human cell lines reveals low levels of viral backbone gene transcription alongside very high levels of SARS-CoV-2 S glycoprotein gene transcription. Genome Med. 2021;13(1):43.
[3] McGonagle D, De Marco G, Bridgewood C. Mechanisms of Immunothrombosis in Vaccine-Induced Thrombotic Thrombocytopenia (VITT) Compared to Natural SARS-CoV-2 Infection. J Autoimmun. 2021;121:102662.
[4] Thiele T, Ulm L, Holtfreter S, Schönborn L, Kuhn SO, Scheer C, Warkentin TE, Bröker B, Becker K, Aurich K, Selleng K, Hübner NO, Greinacher A. Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood. 2021 May 14;138(4):299–303.doi: 10.1182/blood.2021012217.
[5] Cines D.B, Bussel J.B. SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N Engl J Med. 2021;384(23):2254-6.