In this issue of Blood, Henden et al uncover a key role for interferon λ (IFN-λ; interleukin 28 [IL-28]/IL-29) in preventing intestinal tissue injury during acute graft-versus-host disease (aGVHD).1
Although the immune-mediated graft-versus-leukemia (GVL) response is paramount for the therapeutic effect of allogeneic hematopoietic stem cell transplantation (allo-HSCT), aGVHD represents a major and potentially fatal clinical complication. Common approaches to alleviate aGVHD have thus far focused on inducing immune tolerance by directly targeting immune cells.2 However, there is now growing evidence that protecting key target tissues such as the gastrointestinal (GI) tract from immune-mediated damage holds great potential for reducing aGVHD severity. In line with this concept, Henden et al propose IFN-λ therapy as a novel strategy to boost tissue protection instead of focusing exclusively on alloreactive immune cells. They present compelling evidence that IFN-λ limits intestinal stem cell (ISC) loss and promotes regeneration of the gut epithelia, thus preserving mucosal barrier function.
The GI tract plays a pivotal role in aGVHD. Damage to the GI epithelia during the conditioning regimen (ie, radiation, chemotherapy) fuels a vicious circle driven by danger signals and the subsequent activation of antigen-presenting cells (APCs). Activated APCs then prime alloreactive donor-derived T cells, which further expand and contribute to tissue injury.2 In recent years, substantial progress has been made in understanding the pathways involved with intestinal regeneration and tissue protection during aGVHD, which include IL-22, type I interferons, keratinocyte growth factor, and the Wnt-agonist R-spondin1.3,4 The administration of recombinant IL-22, for instance, drives intestinal epithelial regeneration via targeting of ISCs4 and is currently being tested in a clinical trial together with systemic corticosteroids in patients with aGVHD (NCT02406651). However, as IL-22 has both protective and inflammatory properties (ie, it has been implicated in cutaneous GVHD), the use of IL-22 in clinical practice must be carefully and cautiously evaluated.5 This concern does not appear to be the case for IFN-λ, whose cytoprotective effects are more specific to the GI tract. The much higher levels of the IFN-λ receptor in the intestinal epithelia in comparison with that in the liver may explain the selective GI changes.
The study by Henden et al also shows that treatment with IFN-λ does not impair alloreactive immune responses mediating GVL because IFN-λ did not affect T-cell function, although it did result in lower T-cell–engraftment rates. The authors attribute the effect on T-cell engraftment to direct effects of IFN-λ on recipient natural killer (NK) cells. Even though they show a clear impact of IFN-λ on the survival and cytotoxic function of NK cells, more needs to be learned about the mechanisms by which these cells limit T-cell expansion after bone marrow transplantation (BMT). Direct effects of IFN-λ on the hematopoietic compartment are expected, due to the expression of functional IFN-λ on B cells, some dendritic cell subsets, or neutrophils.6 The finding that IFN-λ attenuates intestinal inflammation through decreased oxygen species production by neutrophils7 may be important in GVHD pathology, given the crucial role of these cells in promoting GI damage.8
The therapeutic exploitation of IFN-λ for aGVHD appears to be easily translatable to clinical application since a pegylated form of IFN-λ (PEG-rIL-29) is already being used in a phase 3 clinical trial for hepatitis C virus infection (NCT01866930). The influence of IFN-λ in viral control extends to a wide variety of viruses such as influenza A virus, SARS coronavirus, and respiratory syncytial virus in the lung, as well as to norovirus, reovirus, and rotavirus in the GI tract.9 In all of these viral infections, an antiviral state is induced in epithelial cells,6 but these changes may be counterproductive with chronic IFN-λ exposure or if tissue damage is already present.10 In the case of aGVHD, Henden et al propose administration of IFN-λ early after allo-HSCT to allow for tissue protection without compromising immune responses associated with beneficial GVL effects. Taken together, this study elegantly addresses the unmet challenge of tissue protection and repair in patients undergoing allo-HSCT. The findings may also impact other disorders that present with GI mucosal tissue damage.
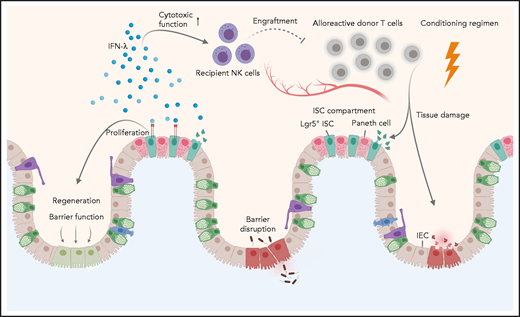
As part of the conditioning regimen, irradiation or chemotherapy lead to tissue damage and barrier dysfunction of the intestinal epithelia, which is further exacerbated by alloreactive donor T cells. Henden et al propose here a dual mechanism for IFN-λ in mitigating acute intestinal GVHD, acting as: (1) a critical proliferative factor for Lgr5+ ISCs, thereby restoring barrier function and regeneration of intestinal epithelial cells (IECs), and (2) a crucial mediator of recipient NK-cell function, thereby ameliorating alloreactive donor T-cell responses.
As part of the conditioning regimen, irradiation or chemotherapy lead to tissue damage and barrier dysfunction of the intestinal epithelia, which is further exacerbated by alloreactive donor T cells. Henden et al propose here a dual mechanism for IFN-λ in mitigating acute intestinal GVHD, acting as: (1) a critical proliferative factor for Lgr5+ ISCs, thereby restoring barrier function and regeneration of intestinal epithelial cells (IECs), and (2) a crucial mediator of recipient NK-cell function, thereby ameliorating alloreactive donor T-cell responses.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal