Abstract
Background: Acute myeloid leukemia (AML) is rare but accounts for 20% of leukemia in the pediatric, adolescent and young adult (AYA) population. Five-year survival ranges widely from 22-90% based on subtype and cytogenetic abnormalities. The risk stratification of AML continues to evolve with increased recognition of inferior prognostic factors in the adult population; however, there has been a lack of similar progress in pediatrics. The DNA methyltransferase 3A (DNMT3A) mutation, most frequently at arginine 882 (DNMT3A mut) is observed in 14-34% of adult AML patients and is associated with inferior outcomes. This mutation shows evidence of anthracycline resistance, which may contribute to its poor prognosis as standard induction therapy for AML consists of a backbone of anthracyclines and purine analogs. Overall survival (OS) and disease-free survival (DFS) are improved in adult patients with DNMT3A mutation who receive consolidative allogeneic hematopoietic stem cell transplant (allo-HSCT). DNMT3A mutation has also been reported in the pediatric and AYA AML in 0-1.5% of cases with unclear prognostic implications. In case its presence in this patient population confers inferior outcomes similar to those seen in adults, these patients may also benefit from allo-HSCT
Objective: Describe the outcome of pediatric and AYA AML patients with DNMT3A mutations
Methods: This study was approved by the institutional review board at the University of Texas at MD Anderson Cancer Center. All patients aged ≤25 years, diagnosed with AML who underwent treatment at our institution between May 1st, 2014 and November 30 th 2020 were screened. Patients who did not undergo testing for DNMT3A mutation were excluded. Outcomes for patients with and without the DNMT3A mutation who underwent allo-HSCT were compared.
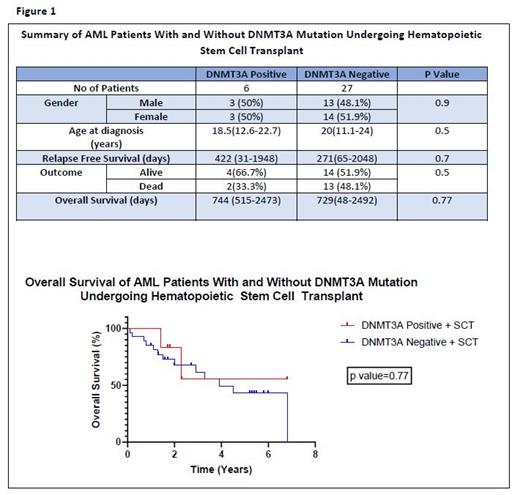
Results: One hundred and five patients were diagnosed with AML during the study period. Forty-five patients were not tested for the DNMT3A mutation and were excluded. Sixty patients were tested for the DNMT3A mutation and were included in the analysis. Seven patients (11.7%) (4 males, 3 females) were found to be DNMT3A mutation positive (+ve). Four of them had the R882 missense mutation. Thirty-three patients (33/60; 55%) (16 males, 17 females) underwent allo-HSCT, with 6 of those being DNMT3A+ve. The median age of DNMT3A+ve and DNMT3A negative(-ve) patients undergoing allo-HSCT was 18.5 years (12.6-22.7) and 20 years (11.1-24) respectively (Figure1). Five patients who were DNMT3A+ve underwent allo-HSCT due to disease relapse and 1 patient due to associated FLT3 mutation. Median overall survival was similar in patients with and without DNMT3A mutations who received allo-HSCT at 744 (515-2473) days and 729(48-2492) days respectively (p value =0.77) (Figure 1). Median OS was significantly lower in the one DNMT3A +ve patient who did not undergo allo-HSCT at 430 days compared to those who underwent allo-HSCT (p value=0.01). Two of the six (33.3%) DNMT3A +ve patients who underwent allo HSCT died due to disease relapse (DR). The one DNMT3A patient who did not undergo allo HSCT died due to refractory disease. In the 27 patients who were DNMT3A -ve and received allo-HSCT, 13 died (13/27;48.1%), 6 from disease relapse (6/27; 22.2%), 2 due to multiorgan failure (2/27; 7.4%) and 5 unknown (5/27;18.5%). Relapse-free survival (RFS) was not significantly different between DNMT3A+ve and DNMT3A -ve patients who underwent allo-HSCT at 422 (31-1948) days and 271 (65-2048) days respectively (p value = 0.7).Of the four patients with the R882 missense mutation, 2 died due to DR (one patient at 192 days post allo-HSCT, one patient who did not undergo allo-HSCT at 352 days after achieving clinical remission one(CR1). Of the 3 patients with non R882 mutations, one died due to DR at 98 days post allo-HSCT.
Conclusion: DNMT3A mutations, although rare in AML, may be associated with poor prognosis and impact risk stratification. This study suggest that consolidative allogeneic HSCT is a reasonable management consideration for this subgroup of patients. DNMT3A is not included in the current AML risk stratification of pediatric and young adult patients and further research is needed to determine the clinical significance of DNMT3A mutations in pediatric and AYA patients with AML and the impact of upfront allo-HSCT.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal