Abstract
Background:
Patients (pts) with cancer have increased morbidity and mortality associated with the development of SARS-Co-V2 infection. The mRNA vaccines BNT162b2 and mRNA-1273 have robust safety and efficacy with 95-97% prevention of severe COVID-19 disease and development of protective antibody titers in 92 - 100% of healthy individuals. By contrast, some pts with hematological malignancy fail to produce anti-spike antibodies (Ab) despite full courses of vaccination. This is particularly true for pts with non-Hodgkin lymphomas (NHL) and chronic lymphocytic leukemia (CLL) who are actively treated with or have received B cell-directed therapies (BCT). We recently demonstrated that NHL pts who received the COVID-19 vaccine within 9 months from BCT demonstrated markedly lower rates of seroconversion (11%) compared to healthy individuals (100%) or a cohort of older (age >65y) residents of a nursing home (91.5%). NHL pts who had received BCT more than 9 months before the vaccine responded more robustly (88%) (Ghione et al, Blood 2021). Here, we update the results of our earlier study and perform an analysis to identify factors that may help predict for adequate response to COVID-19 vaccines. We hypothesized that neutrophil (N) or lymphocyte (L) counts and/or N/L ratio (NLR) at baseline might predict for adequate Ab production in response to COVID-19 vaccines after receipt of BCT.
Methods:
This was an observational study performed at Roswell Park Comprehensive Cancer Center. Pts with NHL/CLL who had received COVID-19 mRNA vaccine were included, vaccine response was assessed as previously described (Ghione et al, Blood 2021). For NLR calculation, pts with CLL or NHL with blood involvement were excluded. Clinical variables were analyzed with the t test and Fisher's exact test; validity and cut-off for the L count was obtained with the ROC analysis using GraphPad9 and SPSS.
Results:
A total of 142 pts with various types of NHL and CLL receiving standard of care treatments were enrolled. Five pts with prior exposure to COVID-19 infection were excluded from the analysis, reaching a total n=137.
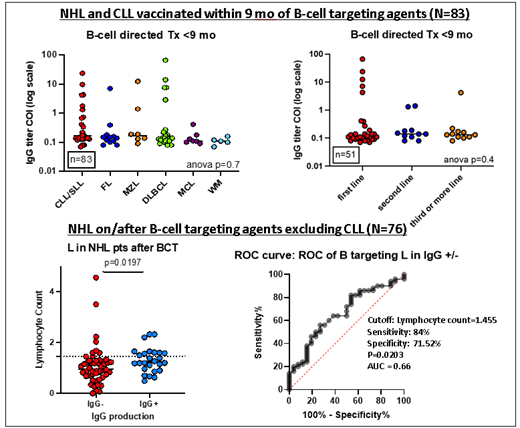
Of 83 pts with NHL (n=57) and CLL(n=26) in our cohort who were vaccinated within 9 months of BCT, 14 (17%) seroconverted. Baseline N count (p= 0.5), sex (p= 0.2), age (p= 0.8), number of prior lines of treatment (p= 0.4), type of disease (p= 0.7), time from end of BCT to vaccine (p= 0.7) and type of vaccine (p= 0.08), did not affect the rate of seroconversion.
For analysis of N and L counts, 37/137 pts with CLL/NHL involving peripheral blood were excluded. Among the remaining 100 pts, 76 had received/were receiving BCT and 24 were either on observation or were on a treatment not including BCT. Only 26/76 (34%) pts on treatment/previously treated with BCT mounted IgG Ab response, while 22/24 (91.6%) patients who were not on BCT mounted the IgG Ab response (p= 0.007). In these NHL pts (N=100), higher L counts and higher NLR were associated with an increased IgG response to the vaccine (p= 0.019). For pts on BCT (N=76) a higher L count (cut-off of 1455 lymphocytes per cubic millimeter of blood [µL] as noted in the ROC) was associated with a higher rate of COVID-19 vaccine response (p= 0.020, with a sensitivity of 84% and specificity 71.5%).
Conclusion:
In this study, we confirm that pts with NHL/CLL receiving COVID-19 vaccination while on active treatment or within 9 months of treatment with BCT respond poorly to COVID-19 mRNA vaccination (17% seroconversion). Although a higher lymphocyte count and NLR ratio were associated with improved seroconversion rates, these were not powerful predictors. Further study of specific lymphocyte sub-populations that contribute to effective vaccine induced immunity in the context of BCT is ongoing. These studies will help to define optimal strategies for immunization in patients receiving BCT.
Torka: TG Therapeutics: Membership on an entity's Board of Directors or advisory committees. Griffiths: Takeda Oncology: Consultancy, Honoraria; Alexion Pharmaceuticals: Consultancy, Research Funding; Abbvie: Consultancy, Honoraria; Novartis: Honoraria; Taiho Oncology: Consultancy, Honoraria; Celgene/Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Boston Biomedical: Consultancy; Astex Pharmaceuticals: Honoraria, Research Funding; Genentech: Research Funding; Apellis Pharmaceuticals: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal