Abstract
Background: Despite significant advances in therapeutic options for multiple myeloma (MM) patients, there is an ongoing need to identify effective treatment strategies in the relapsed space. The efficacy of daratumumab, pomalidomide and dexamethasone (DPD) in relapsed and/or refractory patients has been demonstrated in clinical trials, but there is limited data at first relapse in a real world setting. Here, we present a retrospective analysis utilizing our institutional data of multiple myeloma patients treated with DPD at first relapse at the Winship Cancer Institute of Emory University.
Methods: Ninety relapsed and/or refractory myeloma (RRMM) patients were identified who had received only one prior line of therapy and subsequently treated with DPD at first relapse. Dose-adjustments were made based on the treating physician's discretion and patient tolerability. Demographic and outcomes data for the patients were obtained from our IRB approved myeloma database and responses were evaluated per IMWG Uniform Response Criteria.
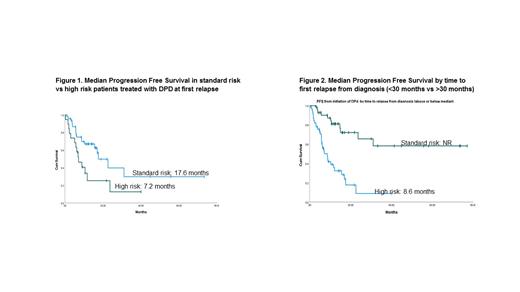
Results: The median age of this cohort was 61.9 years (range, 38-85). Other notable patient characteristics include: M/F 44.4%/55.6%; W/AA/Asian 50%/46.7%/3.3%; ISS I/II/III 28.9%/31.1%/20%; Isotype IgG/IgA/FLC 62.2%/17.8%/16.7%; standard risk/high risk 21.1%/52.2%. High risk disease was defined as the presence of t(4;14), t(14;16), del(17p), and/or complex karyotype. A total of 69 patients (76.7%) underwent autologous stem cell transplant (ASCT) upfront after attaining at least a partial response with induction therapy. The most common induction regimen was RVD (78.9%). 81.1% of patients received maintenance therapy, with 50.5% receiving single-agent lenalidomide maintenance and 72.2% receiving a lenalidomide-based maintenance regimen (RVD: 8 pts; Rd: 4 pts; IRD: 7 pts, KRD: 1 pt). With a median follow up of 72 months, the median OS from diagnosis was 158.6 months (95% CI 126.7-190.5) for the entire cohort. The median PFS from time of initiation of DPD was 15.6 months (95% CI 9.9-21.2), and the median OS from time of initiation of DPD was 41.3 months. For high risk vs standard risk patients, the mPFS from time of initiation of DPD was 7.2 months (95% CI 3.6-10.7) vs 17.6 months (95% CI 10.9-24.3), respectively. Median PFS2 in patients <2 years and >2 years from transplant was 8.6 months vs NR, respectively.
Conclusions: These results illustrate the activity of DPD at first relapse in a predominantly len-refractory RRMM cohort of patients with impressive long-term outcomes. This benefit was particularly demonstrated in patients with time to relapse of >2 years post-transplant.
Joseph: GSK: Honoraria; BMS: Research Funding; Takeda: Research Funding; Karyopharm: Honoraria. Boise: AstraZeneca: Honoraria, Research Funding; AbbVie/Genentech: Membership on an entity's Board of Directors or advisory committees. Hofmeister: BlueBird Bio: Other: Non-CME speaker; Aptitude Health: Other: Non-pharma speaker for education, research, marketing; Verascity: Other: Non-pharma speaker for education, research, marketing; TRM Oncology: Other: Non-pharma speaker for education, research, marketing; DAVA Oncology: Other: Non-pharma speaker for education, research, marketing; Medscape: Other: Non-pharma speaker for education, research, marketing; Amgen: Other: Non-CME speaker; BMS: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Philips Gilmore: Other: CME speaker; Non-pharma speaker for education, research, marketing; BioAscend: Other: CME speaker; Imbrium: Membership on an entity's Board of Directors or advisory committees; Myeloma360: Membership on an entity's Board of Directors or advisory committees; Genzyme: Membership on an entity's Board of Directors or advisory committees; Takeda: Other: Local PI of CST; Oncolytics: Other: National PI for CST; PI or co-PI IST; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Other: Local PI of CST; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Local PI of CST; Nektar Therapeutics: Membership on an entity's Board of Directors or advisory committees, Other: Local PI of CST; BMS/Celgene: Other: National PI for CST; PI or co-PI IST; Local PI of CST; Sanofi: Other: National PI for CST; PI or co-PI IST; Ohio State University: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: IP rights, Patents & Royalties. Kaufman: Incyte, TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Sutro, Takeda: Research Funding; Roche/Genetech, Tecnopharma: Consultancy, Honoraria; Fortis Therapeutics: Research Funding; Heidelberg Pharma: Research Funding; BMS: Consultancy, Research Funding; Amgen: Research Funding; Tecnofarma SAS, AbbVie: Honoraria; Incyte, celgene: Consultancy; Janssen: Honoraria; Novartis: Research Funding; Genentech, AbbVie, Janssen: Consultancy, Research Funding. Lonial: Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; GlaxoSmithKline: Consultancy, Honoraria, Research Funding; Merck: Honoraria; AMGEN: Consultancy, Honoraria. Nooka: GlaxoSmithKline: Consultancy, Other: Travel expenses; Amgen: Consultancy, Research Funding; Oncopeptides: Consultancy; Janssen Oncology: Consultancy, Research Funding; Sanofi: Consultancy; Karyopharm Therapeutics: Consultancy; Takeda: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy; Adaptive technologies: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal