Abstract
Background:
Myelofibrosis (MF) is a lethal hematological malignancy associated with somatic mutations in JAK2, CALR or MPL. Ruxolitinib is the first JAK1/2 inhibitor approved for treatment of MF. Ruxolitinib does not prevent disease progression and thus, allogeneic hematopoietic stem cell transplantation (HSCT) remains the recommended therapy for eligible patients treated with curative intent. Ruxolitinib discontinuation, in preparation for HSCT is challenging as patients experience return of symptoms/splenomegaly. Therefore, ruxolitinib is often continued during and after HSCT in an off-label fashion, yet little is known about the safety of this approach. In addition, ruxolitinib is now utilized to treat steroid refractory acute and chronic graft versus host disease (GVHD) irrespective of underlying disease.
Methods:
This is a phase II, multi-center, investigator-initiated trial investigating ruxolitinib given pre-, during- and post-HSCT for patients with primary or secondary MF (NCT03427866). The study utilizes ruxolitinib during and after HSCT in MF patients for one year after HSCT. The accrual goal is 48 patients with 1-year GVHD free and relapse free survival (GRFS) as the primary endpoint. Secondary endpoints include overall and progression free survival, engraftment and incidence of acute and chronic GVHD, respectively. Patients are treated with reduced intensity conditioning with fludarabine (30mg/m 2/day x 5 days) and melphalan (100mg/m 2 or 140mg/m 2 x 1). HSCT grafts are with 7/8 or 8/8 HLA-matched peripheral blood stem cells with tacrolimus and methotrexate as standard GVHD prophylaxis.
Results:
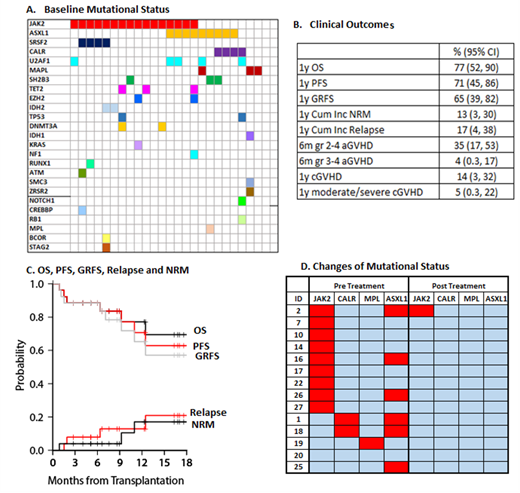
This pre-planned interim analysis includes 26 MF patients who underwent HSCT between September 2018 and January 2021. An interim analysis was included in the trial design to ensure safety of this approach midway through accrual. Median age was 66 (range, 46-75) and 65% were male. 88% had 8/8 matched related grafts, and 92% had intermediate-2 or high DIPSS risk at the time of transplant. 14 (54%) patients were previously treated with ruxolitinib. At HSCT, 58% had JAK2, 12% CALR, 12% MPL, and 35% ASXL1 mutations (Figure A). There were no unexpected toxicities related to ruxolitinib therapy. The most common grade 3/4 hematologic adverse events (AE) were anemia (n=4), thrombocytopenia (n=3). There were few observed grade 3/4 non hematologic AEs and included infection (n=2) and hypertriglyceridemia (n=1). Median time to neutrophil engraftment was 15 days (range 11-38) after HSCT. All but one patient achieved successful neutrophil engraftment. Median day 30 donor all cell chimerism was 100% (range 95-100). Clinical outcomes are summarized in Figure B. With median follow-up among survivors of 12 months (range 3-24), 1-yr GRFS was 65%. OS, PFS, and cumulative incidence of NRM and disease relapse were 77%, 71%, 13% and 17%, respectively (Figure C). There was no grade IV acute GVHD and only one case of grade III acute GVHD. Cumulative incidence of all chronic GVHD and moderate-severe chronic GVHD was 14% and 5%, respectively. There was no severe chronic GVHD and only one patient developed moderate chronic GVHD.
As part of the study, next generation sequencing (NGS) was obtained pre- and 100 days post-HSCT. 14 patients have paired samples, including 6 with ASXL1 mutations. All but one patient, who remains in remission at last follow up, no longer had mutations detected by NGS at day 100 (Figure D). Ongoing studies will assess for the presence of low-level mutation not detectable by clinical NGS testing.
Discussion:
The interim results of our multicenter study demonstrate safety of ruxolitinib administration pre, during and post-HCT with very favorable engraftment rates and no unexpected toxicities of ruxolitinib use. In addition, we demonstrate superior PFS, OS and GRFS compared to historical observations. Incidence of severe acute and chronic GVHD are thus far minimal, indicating excellent GVHD control with prophylactic and continued ruxolitinib use.
Hobbs: Bayer: Research Funding; Incyte Corporation: Research Funding; Celgene/Bristol Myers Squibb: Consultancy; AbbVie.: Consultancy; Merck: Research Funding; Novartis: Consultancy; Constellation Pharmaceuticals: Consultancy, Research Funding. Byrne: Karyopharm: Research Funding. Defilipp: Incyte Corp.: Research Funding; Regimmune Corp.: Research Funding; Omeros, Corp.: Consultancy; Syndax Pharmaceuticals, Inc: Consultancy. Chen: Gamida: Consultancy; Incyte: Consultancy.
Will describe the use of ruxolitinib in the ongoing clinical trial.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal