Abstract
Background: Cytomegalovirus (CMV) infection is a major and potentially life-threatening complication after allogenic hematopoietic stem cell transplantation (allo-SCT). Adoptive transfer of CMV-specific T cells (CTL) from original transplant donor or third-party donor has both emerged as an effective method for CMV infection after allo-SCT. The potential advantages of third party CTL versus transplant donor CTL includes that it is not limited by donor viral immune and can be banked in advance for clinical use. However, as the expansion and persistent of transfused third-party CTL in vivo was considered to be shorter when compared with donor CTL, can third-party CTL provide comparable long-term antiviral efficacy as transplant donor CTL? In fact, the safety and efficacy of these two kinds of CTL have not been compared directly. In addition, the mechanisms driving sustained antiviral immunity induced by these two kinds of CTL remains to be established and compared.
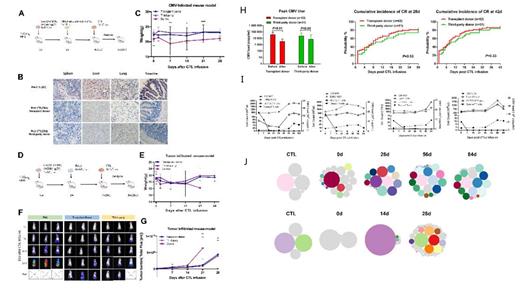
Methods: i) We established humanized CMV-infected mouse model and tumor-infiltration mouse model, and compared the antiviral ability of transplant donor and third-party donor derived CTL for CMV infection in mouse models. We comparatively investigated the in vivo recovery of CMV-specific immunity and analyzed the underlying mechanisms driving sustained antiviral immunity induced by these two types of CTL therapy. ii) We collected data from 31 patients who received third-party CTL and selected matched pairs of 62 patients who received donor CTL for refractory CMV infection after allo-SCT, and compared the safety and efficacy of these two kinds of CTL for CMV infection in clinical patients. We also track the infused CTL populations and evaluated the recovery of virus-specific immunity in clinical patients after donor and third-party CTL therapy.
Results: i) In mouse models, we observed that adoptively infused donor and third-party CTL could both migrated to the virus or tumor infiltration organs, and contributed to comparable diminishing in CMV pathology and viral burden in target organs. The kinetics of CMV-specific immunity recovery was comparable in donor and third-party CTL group, which further conferred the comparable antiviral response of these two kinds of CTL for CMV infection. When performed a detailed analysis of the recovered source of CTL, we observed a preferential proliferation and expansion of graft-derived endogenous CTL in both donor and third-party CTL therapy group. ii) In clinical patients, adoptive therapy with donor or third-party CTL had comparable clinical response without significant therapy-related toxicity. The cumulative CR rates at 4 th weeks after CTL infusion was 80.6% in donor group and 83.1% in third-party group. We also observed strong expansion of CD8 + tetramer + T cells both after donor and third-party CTL infusion, which were associated with a reduced or cleared viral load.
Conclusion:Adoptive therapy with transplant donor or third-party CTL had comparable antiviral response for CMV infection by promoting the restoration of CMV-specific immunity. Our data suggest that both transplant donor or third-party CTL may stimulate the recovery of graft-derived endogenous CMV-specific immunity.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal